Session Information
Session Type: Abstract Submissions (ACR)
Table. Baseline disease characteristics
|
Mean (SD) |
Rituximab-US |
Rituximab-EU n=74 |
n=73 |
|
Swollen joint count (28) |
14.1 (5.92) |
13.0 (6.53) |
11.7 (5.36) |
|
Tender/painful joint count (28) |
18.1 (6.45) |
14.9 (6.79) |
14.6 (6.70) |
|
Swollen joint count (66) |
19.3 (8.73) |
17.8 (10.56) |
15.6 (8.91) |
|
Tender/painful joint count (68) |
30.4 (15.33) |
23.3 (13.23) |
22.9 (12.46) |
|
HAQ-DI score |
1.75 (0.62) |
1.59 (0.54) |
1.65 (0.57) |
|
Serum hsCRP (mg/L) |
18.17 (24.80) |
14.80 (17.42) |
12.70 (15.28) |
|
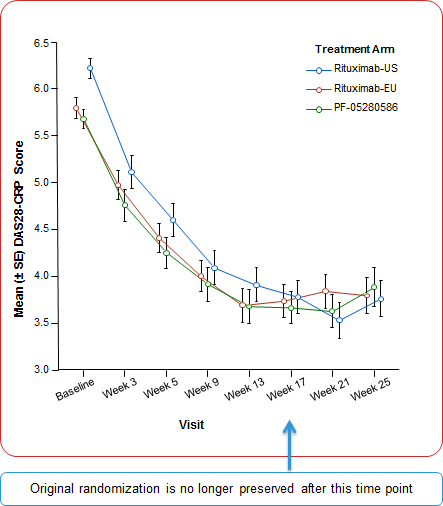
DAS28-CRP |
6.22 (0.89) |
5.79 (0.95) |
5.68 (0.86) |
hsCRP=high-sensitivity C reactive protein; DAS28-CRP=Disease Activity Score in 28 joints – C-reactive protein; HAQ-DI=Health Assessment Questionnaire – Disability Index; SD=standard deviation
Disclosure:
J. C. P. Becker,
Pfizer Inc.,
1,
Pfizer Inc.,
9;
D. Yin,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
L. A. Melia,
Pfizer Inc.,
3;
R. Li,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
B. Gumbiner,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
D. Thomas,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
G. Spencer-Green,
Pfizer Inc.,
3;
X. Meng,
Pfizer Inc.,
3.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-i-trial-comparing-pf-05280586-a-potential-biosimilar-and-rituximab-in-subjects-with-active-rheumatoid-arthritis/