Session Information
Date: Sunday, November 13, 2016
Title: Metabolic and Crystal Arthropathies - Poster I: Clinical Practice
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Data generated by Phase 1 and 2 studies suggest that the extended-release (XR) formulation of febuxostat (FBX) may provide equal or better reduction in serum urate level (sUA) in patients (pts) with gout, with reduced exposure (Cmax and AUC), compared with an immediate-release (IR) formulation. This Phase 2 study was conducted to evaluate the efficacy and safety of FBX XR compared with FBX IR in pts with gout and moderate renal impairment.
Methods: In a Phase 2, multicenter, randomized, placebo-controlled, double-blind study, pts with gout (sUA ≥8.0 mg/dL, moderate renal impairment [estimated glomerular filtration rate ≥30 mL/min and <60 mL/min], and ≥1 gout flare within the previous 12 months) received placebo or FBX XR 40 mg, XR 80 mg, IR 40 mg, or IR 80 mg once daily for 3 months. The primary endpoint was the proportion of pts with sUA <5.0 mg/dL at Month 3. Secondary endpoints were proportions of pts with at least 1 flare requiring treatment during the 3-month treatment period and of pts with sUA <6.0 mg/dL at Month 3.
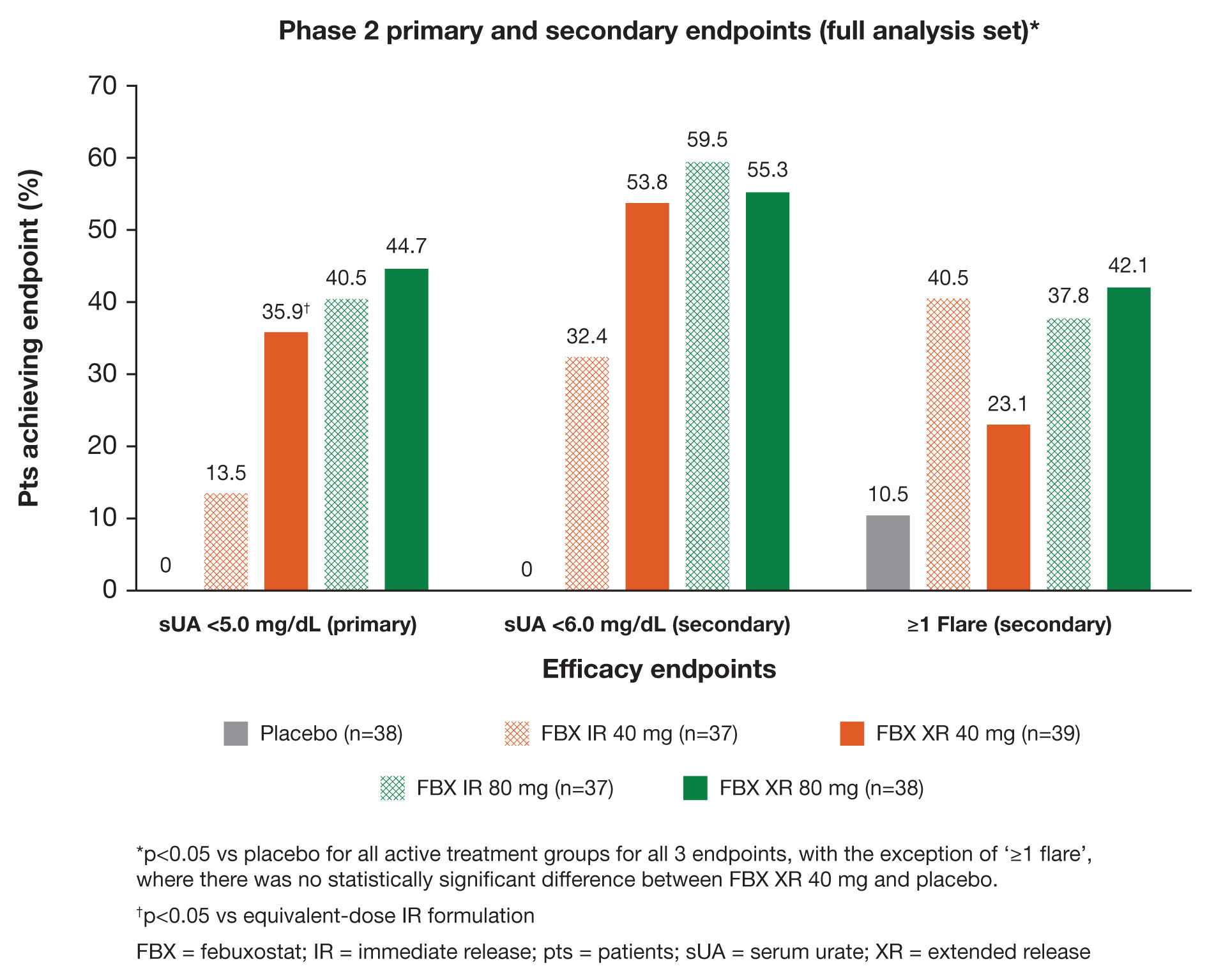
Results: A total of 189 pts received treatment with placebo (n=38) or FBX XR 40 mg (n=39), XR 80 mg (n=38), IR 40 mg (n=37), or IR 80 mg (n=37). A higher proportion of pts receiving FBX XR 40 mg achieved sUA <5.0 mg/dL versus (vs) IR 40 mg at Month 3 (35.9% vs 13.5%, respectively; p=0.034). All FBX groups showed a higher proportion of pts with at least 1 flare during treatment compared with placebo-treated pts, but a smaller proportion of pts in the FBX XR 40-mg group experienced flares compared with IR 40 mg (23.1% vs 40.5%, respectively). Additionally, a numerically higher proportion of pts achieved sUA <6 mg/dL with FBX XR 40 mg compared with IR 40 mg (53.8% vs 32.4%, respectively). FBX XR 80 mg did not differentiate from IR 80 mg for the primary or secondary endpoints. Primary and secondary endpoint results are shown (Figure). Treatment-emergent and treatment-related adverse events were infrequent and generally did not differ among treatment groups.
Conclusion: Significantly more pts receiving FBX XR 40 mg achieved the primary endpoint of sUA reduction to <5.0 mg/dL at the Month 3 visit compared with FBX IR 40 mg. There was a trend toward lower flare rates in the FBX XR 40 mg vs the IR 40 mg group. Overall, incidence rates of treatment-emergent and treatment-related adverse events were low.
To cite this abstract in AMA style:
Gunawardhana L, Becker MA, Whelton A, Hunt B, Castillo M, Dong X, Saag K. A Phase 2 Study to Evaluate the Efficacy and Safety of Febuxostat Extended- Versus Immediate-Release Formulations in Patients with Gout and Moderate Renal Impairment [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/a-phase-2-study-to-evaluate-the-efficacy-and-safety-of-febuxostat-extended-versus-immediate-release-formulations-in-patients-with-gout-and-moderate-renal-impairment/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-2-study-to-evaluate-the-efficacy-and-safety-of-febuxostat-extended-versus-immediate-release-formulations-in-patients-with-gout-and-moderate-renal-impairment/