Session Information
Date: Tuesday, November 7, 2017
Title: Systemic Sclerosis, Fibrosing Syndromes and Raynaud's – Clinical Aspects and Therapeutics II
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Systemic sclerosis (SSc) is characterized in part by chronic activation of the innate immune system with fibrosis. Anabasum is a non-immunosuppressive, synthetic, orally administered selective CB2 agonist that activates resolution of innate immune responses in animal models of SSc and healthy humans. This study evaluated safety and efficacy of anabasum in diffuse cutaneous SSc (dcSSc).
Methods: A double-blind, randomized placebo (PBO)-controlled 16-week Phase 2 trial (JBT101-SSc-001) enrolled subjects with dcSSc ≤ 6 years duration on stable medications including immunosuppressive drugs. Subjects received anabasum 5 mg QD, 20 mg QD, or 20 mg BID x 4 weeks, then 20 mg BID x 8 weeks, or PBO x 12 weeks. Subjects were followed off study drug x 4 weeks. The primary efficacy outcome was ACR Combined Response Index in diffuse cutaneous Systemic Sclerosis (CRISS).
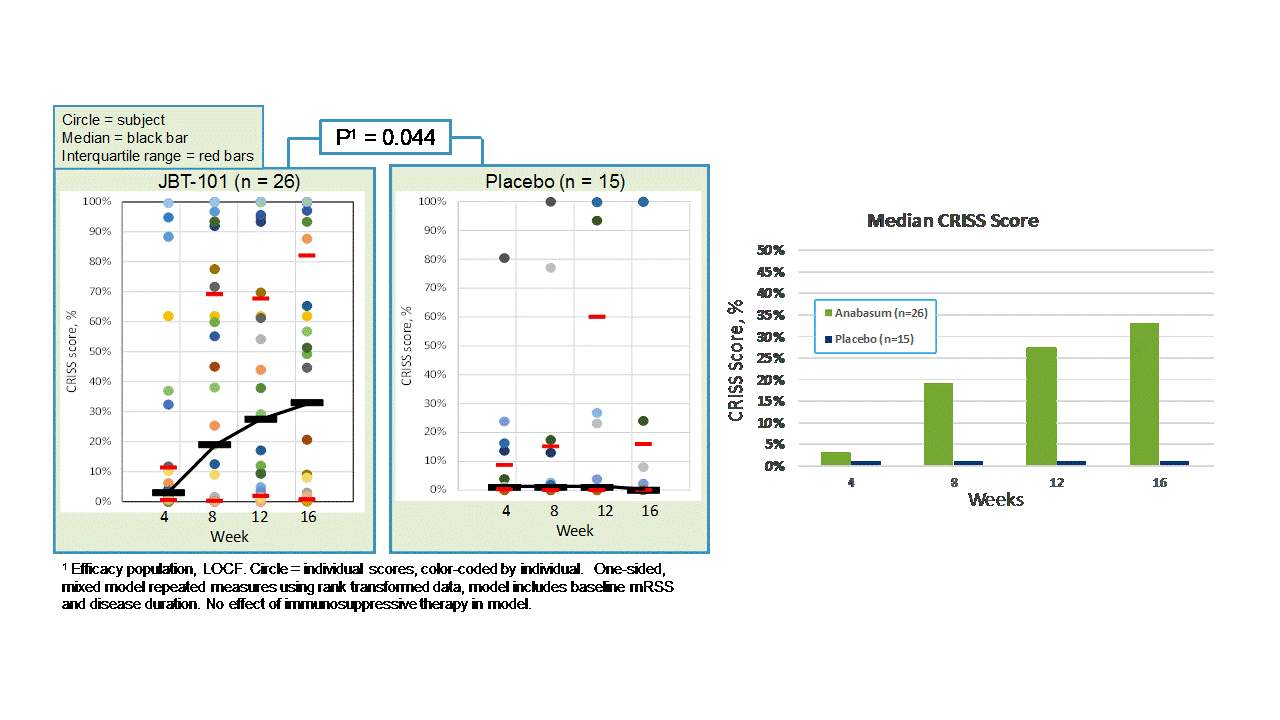
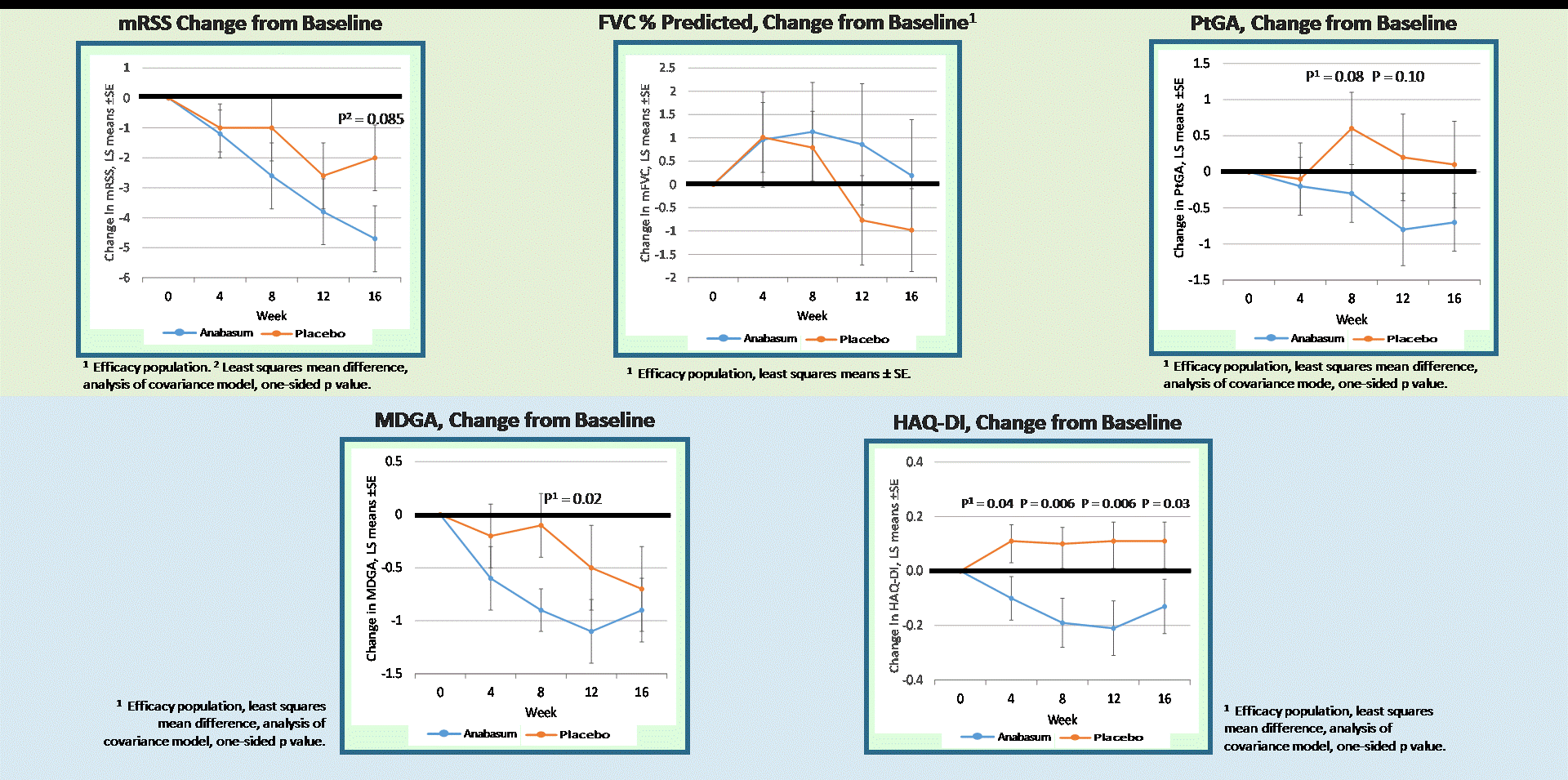
Results: Forty-two subjects received study drug: anabasum N = 27 and PBO N = 15. Baseline patient characteristics were similar in both groups. There were no serious, severe or unexpected adverse events (AEs) related to anabasum. Severity and relationship of AEs to study drug were similar in both groups. AEs in ≥ 10% of anabasum subjects were dizziness and fatigue. Anabasum subjects had greater improvement in ACR CRISS scores than PBO subjects over 16 weeks (Fig. 1, p = 0.044, 1-sided, mixed model repeated measures included baseline mRSS and disease duration). Anabasum subjects had greater improvement and less worsening in individual CRISS core measures including modified Rodnan Skin Score (mRSS), Patient Global Assessment (PtGA), Physician Global Assessment (MDGA), and HAQ-DI (Fig. 2).
Figure 1. CRISS Scores
Figure 2. CRISS Core Measures
There were strong correlations of CRISS scores with change from baseline in mRSS (r = -0.894, p < 0.0001) and MDGA (r =-0.591, P < 0.0001) with weaker correlations with FVC % predicted (r = 0.280, P = 0.0006), PtGA (r = -0.270, P = 0.0008), and HAQ-DI (r = -0.294, P = 0.0003). Patient-reported outcomes of SSc skin symptoms, itch, and PROMIS-29 physical function, pain interference, and sleep also improved (P < 0.05 for all). Evaluation of gene transcripts in skin biopsies showed anabasum but not placebo reduced expression of key genes implicated in SSc and gene ontology pathways associated with inflammation and fibrosis.
Conclusion: Anabasum had acceptable safety and tolerability in this Phase 2 trial in dcSSc and demonstrated consistent evidence of clinical benefit. Changes in gene expression were consistent with biologic effects of anabasum on pathways relevant to SSc. Further evaluation of anabasum in treatment of dcSSc is warranted.
To cite this abstract in AMA style:
Spiera RF, Hummers LK, Chung L, Frech TM, Domsic RT, Hsu V, Furst DE, Gordon JK, Mayes MD, Simms RW, Constantine S, White B. A Phase 2 Study of Safety and Efficacy of Anabasum (JBT-101), a Cannabinoid Receptor Type 2 Agonist, in Diffuse Cutaneous Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/a-phase-2-study-of-safety-and-efficacy-of-anabasum-jbt-101-a-cannabinoid-receptor-type-2-agonist-in-diffuse-cutaneous-systemic-sclerosis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-2-study-of-safety-and-efficacy-of-anabasum-jbt-101-a-cannabinoid-receptor-type-2-agonist-in-diffuse-cutaneous-systemic-sclerosis/