Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Anifrolumab is a fully human, IgG1κ monoclonal antibody that binds to the type I IFN receptor and inhibits activity of all type I IFNs.1 In the MUSE phase 2b randomized controlled trial (Study 1013, NCT01438489), anifrolumab demonstrated an acceptable safety profile and efficacy on a range of clinical endpoints in patients with moderate to severe SLE. An open-label extension (OLE) of Study 1013 (Study 1145, NCT01753193) evaluated long-term safety and tolerability of anifrolumab.
Methods: Study 1145 was a 3-year, multinational OLE in adults with moderate to severe SLE (per ACR classification criteria, assessed in Study 1013) who completed randomized treatment with anifrolumab 1000 or 300 mg or placebo in Study 1013 to Day 337 with follow-up to Day 422. All patients in Study 1145 initially received IV anifrolumab 1000 mg every 4 weeks (Q4W). After data from Study 1013 showed the 300-mg dose had a better benefit/risk profile, the dosage in Study 1145 was amended to 300 mg Q4W. Patients received anifrolumab Q4W over 156 weeks with 85 days of follow-up. The primary objective was to evaluate long-term safety/tolerability. Efficacy, pharmacodynamics, and health-related quality of life (HRQoL) were exploratory objectives. Safety was assessed at every visit; SLEDAI-2K and SLICC Damage Index were measured every 3 and 6 months, respectively.
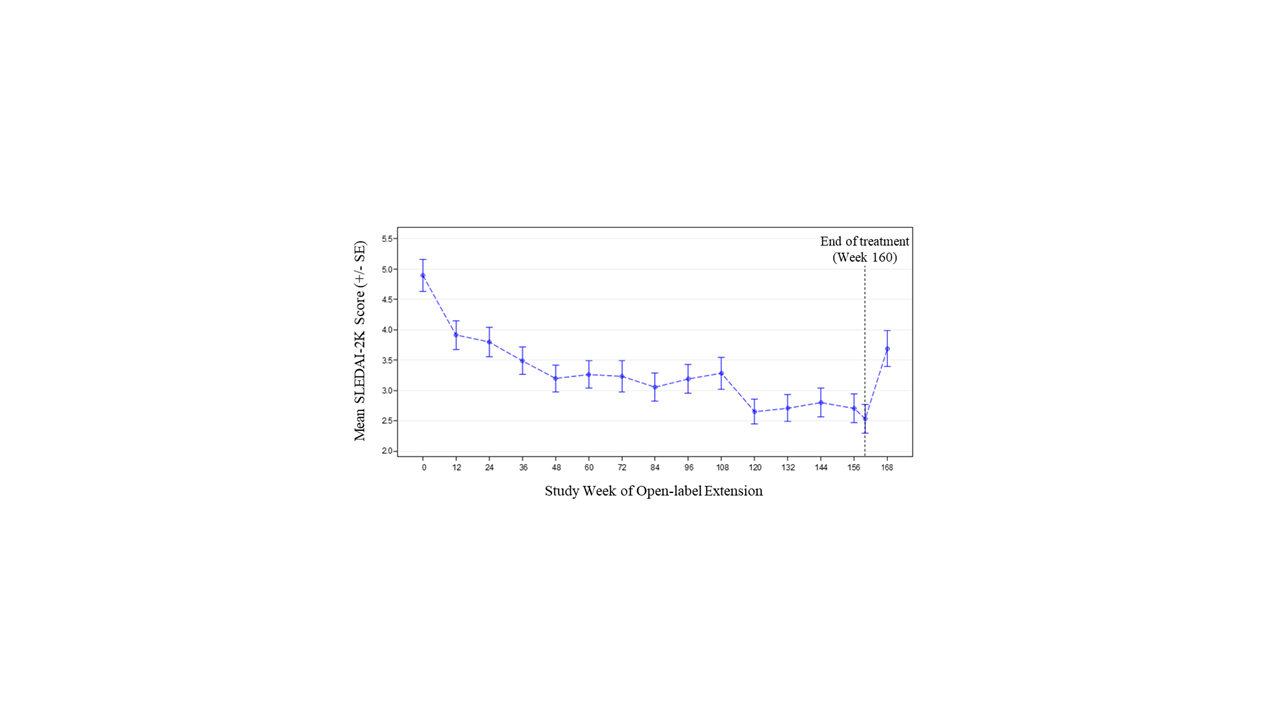
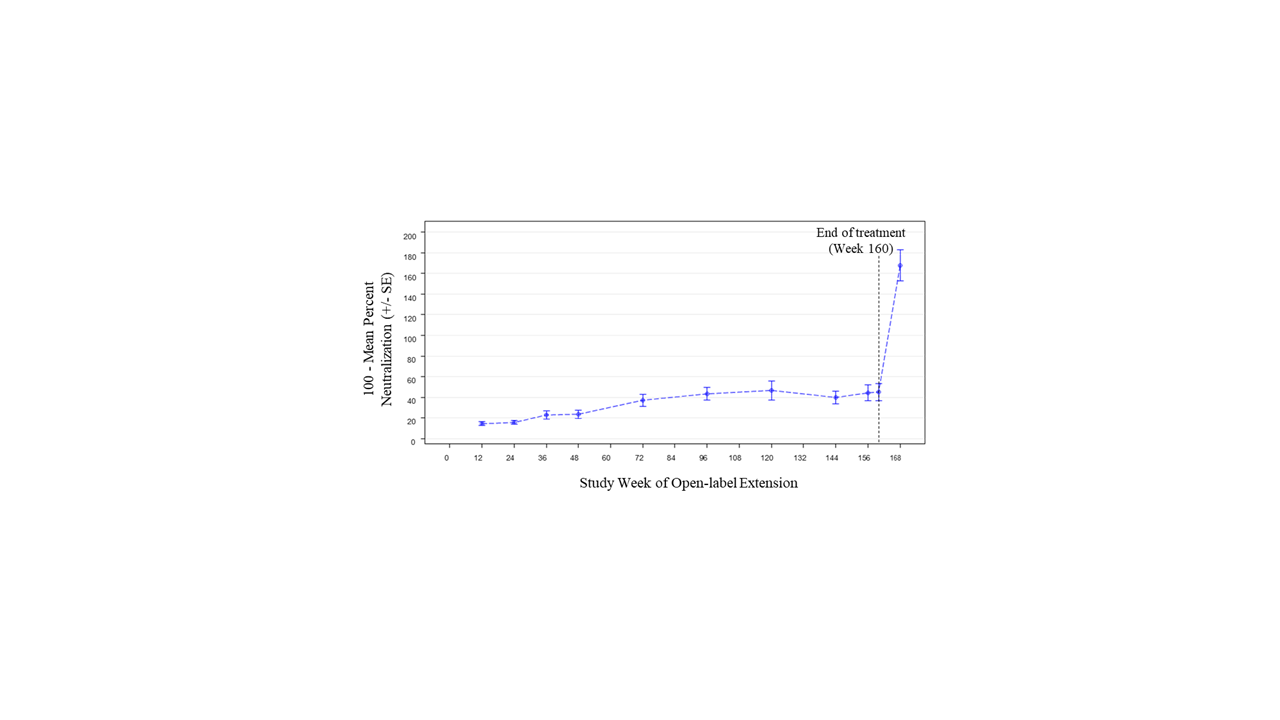
Results: Of 305 randomized patients in Study 1013, 218 (71.5%) at 59 sites participated in Study 1145; 66/218 (30.3%) received placebo in Study 1013. Of 218 patients in Study 1145, 139 (63.8%) completed 3 years of treatment. The most common reason for treatment discontinuation was patient withdrawal (31/218, 14.2%). Treatment was discontinued due to adverse events (AEs) for 15/218 patients (6.9%). During Year 1, 152/218 patients (69.7%) had ≥1 AE (Table). Over the full study period, the rate of serious AEs was 8.56 per 100 patient-years (PY); the most common serious AEs were SLE flares and pneumonia (each 0.70 per 100 PY). The most common AE of special interest was herpes zoster (1.92 per 100 PY). There was 1 death due to pneumonia. Two of 218 patients were anti-drug antibody positive at any time during treatment (both persistently positive). Sustained improvement of the SLEDAI-2K was observed through the end of treatment (mean change –2.1 [SD=3.5] from baseline to Week 160; Figure 1). Short Form 36 Health Survey physical and mental component scores showed similar patterns of sustained improvement. SLICC Damage Index generally remained stable over time (mean change 0.1 [SD=0.6] from baseline to Week 168). Neutralization of type I IFN gene signature (IFNGS) expression was rapid and sustained in patients with high baseline IFNGS (Figure 2). C3, C4, and anti-dsDNA showed numeric trends toward sustained improvement.
Conclusion: Long-term anifrolumab treatment for up to 3 years was generally safe and well tolerated. The safety profile was consistent with the 52-week Study 1013. Disease activity, HRQoL, and SLE-related serology showed sustained improvement.
Writing assistance by Maria Prada, PhD (JK Associates Inc., Fishawack Group of Companies).
Reference
- Riggs JM, et al. Lupus Sci Med. 2018;5:e000261.
To cite this abstract in AMA style:
Chatham W, Furie R, Saxena A, Brohawn P, Schwetje E, Abreu G, Tummala R. A Phase 2, Open-label Extension Study to Evaluate Long-term Safety of Anifrolumab in Adults with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-phase-2-open-label-extension-study-to-evaluate-long-term-safety-of-anifrolumab-in-adults-with-systemic-lupus-erythematosus/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-2-open-label-extension-study-to-evaluate-long-term-safety-of-anifrolumab-in-adults-with-systemic-lupus-erythematosus/