Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Azathioprine (AZA) is used to treat rheumatic diseases and to prevent transplant rejection. There is marked variability in toxicity to azathioprine; one such toxicity is thiopurine-induced acute pancreatitis (TIAP). The cause of TIAP is not known, but a HLA-DRB1*07:01-HLA-DQA1*02:01 haplotype was recently identified as a risk factor, suggesting that pancreatic injury may be immunologically mediated and modified by genetic factors. We aimed to build a cohort of patients who had pancreatic injury while taking AZA to perform pharmacogenetic studies. Here we present preliminary data.
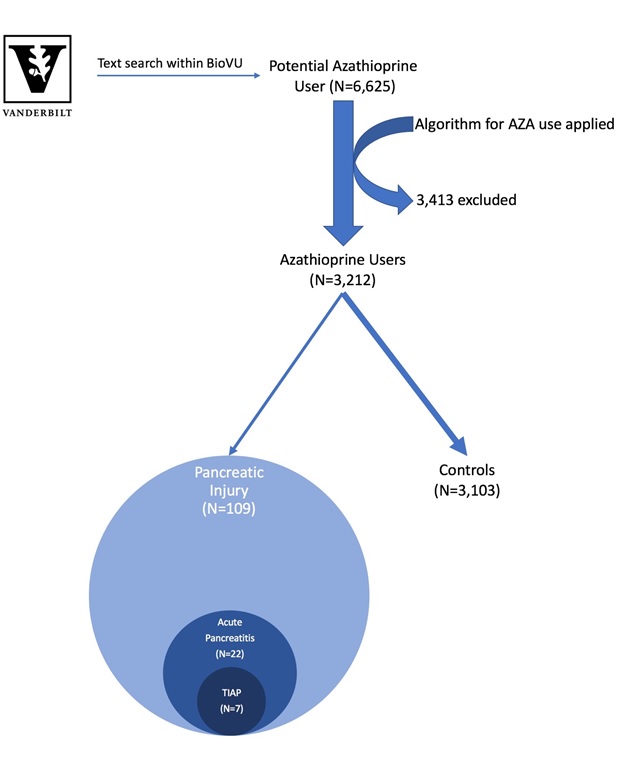
Methods: We used the Vanderbilt University Medical Center BioVU resource that contains de-identified medical records linked to a biobank containing DNA samples from 225,000 patients. A validated bioinformatic algorithm was used to assemble a cohort of European American patients taking AZA. Those AZA users with amylase or lipase values that exceeded twice the upper limit of normal or with ICD-9/ICD-10 codes for acute pancreatitis were identified. Each record was reviewed to confirm AZA use at the time and to further classify patients into three not mutually exclusive categories: pancreatic injury (enzyme abnormalities alone), acute pancreatitis (defined by American College of Gastroenterology Guidelines), or TIAP (Figure 1). The remaining patients served as controls. Age, sex, race, and BMI were compared between groups. All available genotypes from Infinium MEGAEX, a platform for genome wide analysis (GWA), were retrieved. After standard quality control measures with removal of variants with minor allele frequency <0.01, we performed an exploratory GWA with 849,934 variants adjusted for age and sex.
Results: We identified 3,212 AZA users. Evidence of pancreatic injury based on enzyme abnormalities occurred in 109 patients, of whom 22 and 7 met criteria for pancreatitis and TIAP, respectively. The remaining 3,103 were controls (Figure 1). Patients with pancreatic injury were more likely to be male (p=0.002). MEGAEX genotypes were available in 34 cases and 449 controls. Although the results did not reach GWAS statistical significance, variants in two snps, rs4859716 (p=3.01×10-6) and rs4843192 (p=7.13×10-6), differed in patients with pancreatic injury.
Conclusion: These preliminary findings suggest that AZA users with pancreatic injury are more likely to be male and that variants in rs4859716 or rs4843192 may be associated with pancreatic injury. The rs4859716 variant is an intergenic variant near the SHROOM3 gene, necessary for epithelial morphogenesis of the gut, and rs4843192 is an intergenic variant near the FOXL1 gene, a tumor suppressor gene whose activity predicts tumor aggressiveness in pancreatic cancer. Further investigation is warranted into clinical and genetic determinants of pancreatic toxicity with azathioprine.
ADDIN EN.REFLIST
To cite this abstract in AMA style:
Reese T, Hurt S, Octaria R, Dickson A, Anandi P, Kawai V, Birdwell K, Hung A, Stein CM, Feng Q, Chung CP. A Personalized Medicine Approach to Improve the Prediction of Azathioprine-Induced Pancreatic Injury: Preliminary Results [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/a-personalized-medicine-approach-to-improve-the-prediction-of-azathioprine-induced-pancreatic-injury-preliminary-results/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-personalized-medicine-approach-to-improve-the-prediction-of-azathioprine-induced-pancreatic-injury-preliminary-results/