Session Information
Date: Sunday, November 10, 2019
Title: Patient Outcomes, Preferences, & Attitudes Poster I: Patient Reported Outcomes
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: IgG4-related disease (IgG4-RD) causes symptoms, the severity of which vary by organ involvement. The Responder Index (RI) captures physicians’ judgement of disease activity, but no disease-specific patient-reported outcome measure (PROM) exists. We sought to develop and validate the Symptom Severity Index (SSI), a PROM that measures the frequency of IgG4-RD-specific symptoms and associated distress.
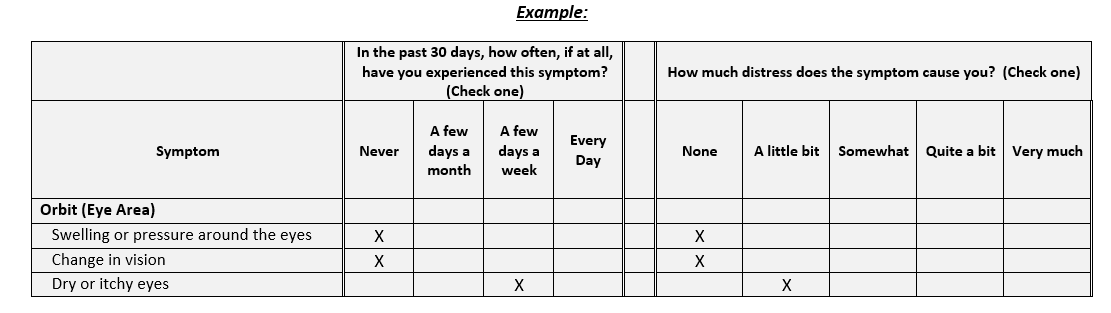
Methods: 40 common IgG4-RD symptoms were identified by three expert clinicians to create a pilot SSI. This was tested in a convenience sample to test usability in a clinic setting and to obtain feedback on the symptom list. Several gaps were identified. Semi-structured qualitative transcribed interviews were then performed with new patients to expand the item set and explore issues that were absent. Newly identified patients described symptoms they attributed to IgG4-RD, associated distress, and other concerns. We reached saturation regarding symptoms and language at 20 patients. The SSI was revised based on these interviews and administered to 5 subjects for feedback on face validity. Minor changes yielded the final SSI which 45 patients completed at routine visits along with SF-36, EQ-5D, and feeling thermometer surveys. For each SSI symptom, a score is calculated by multiplying frequency (range “Never” [0] to “Every Day” [3]) by associated distress (range “None” [0] to “Very Much” [4]). Each symptom score is then summed (e.g., SSI score). We assessed construct validity by measuring known-group and convergent/divergent validity.
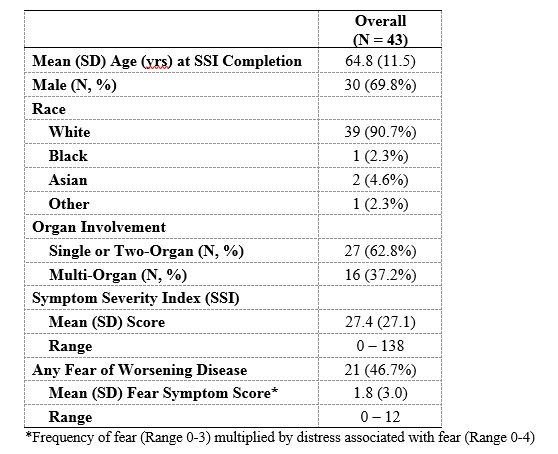
Results: The final SSI assesses the frequency and distress of 24 symptoms in 8 categories: General Health, Orbit, Sino-nasal Area, Salivary Glands, Chest, Abdomen, Skin and Extremities, and Genitourinary (Figure). A question on fear of more serious or severe disease was included based on qualitative interview findings. 45 patients completed the SSI 64 times (Table 1). The mean (SD) SSI score was 27.4 (27.1) and the range was 0-138. Fear of more severe disease was reported by 21 (46.7%) patients. The SSI inversely correlated well with the SF-36 (r= – 0.47, p< 0.001) and the feeling thermometer (r= – 0.50, p< 0.001). The symptom frequency score correlated with the RI (r=0.3, P=0.03) but the total SSI score did not (r=0.2, P=0.1). The SSI inversely correlated weakly with the EQ-5D (r= – 0.26, p=0.09). The fear score inversely correlated with the SF-36 item regarding role limitation due to emotional problems (r= – 0.36, p=0.003). Trends suggested that the median (IQR) SSI score was higher in active vs inactive disease (26 [12, 41] vs. 12 [6, 31]) and multi-organ vs one or two-organ disease (25 [12, 33] vs. 13 [6, 33]).
Conclusion: The SSI is an IgG4-RD-specific PROM developed with patient and clinician input to achieve face and content validity. In addition to symptoms and distress related to organ-specific disease, fear of worsening or more serious disease is common. The SSI has good construct validity in comparison with generic quality of life measures. The symptom frequency score correlated with the RI but the overall SSI score did not, suggesting that the SSI better reflects patient-reported severity. The validity of the SSI should be further investigated in multi-center studies.
To cite this abstract in AMA style:
Harkness T, Donelan K, Fu X, Wallwork R, Perugino C, Stone J, Wallace Z. A Novel Patient-Reported Outcome Measure in IgG4-Related Disease: The Symptom Severity Index [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-novel-patient-reported-outcome-measure-in-igg4-related-disease-the-symptom-severity-index/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-novel-patient-reported-outcome-measure-in-igg4-related-disease-the-symptom-severity-index/