Session Information
Date: Wednesday, November 8, 2017
Title: Metabolic and Crystal Arthropathies II: Mechanisms of Crystal Inflammation and Metabolism
Session Type: ACR Concurrent Abstract Session
Session Time: 9:00AM-10:30AM
A non-coding genetic variant maximally associated with serum urate levels is functionally linked to HNF4A-dependent PDZK1 expression
Background/Purpose: Genome-wide association studies have revealed several dozen genetic variants associated with serum urate levels. These loci are dominated by genes encoding renal and gut uric acid transporters (SLC2A9, ABCG2, SLC17A1-4, SLC22A11, SLC22A12) and auxiliary molecules such as PDZK1. The precise molecular mechanisms by which causal genetic variant(s) affect serum urate are largely unknown, although the majority of influence will be through control of gene expression. Here we functionally link the maximally associated genetic variant (rs1967017) at the PDZK1 locus to elevated PDZK1 expression via the HNF4A transcription factor, revealing a new mechanism for serum urate control.
Methods: We performed expression quantitative trait locus (eQTL) and likelihood analyses (PAINTOR and COLOC) followed by gene expression assays in zebrafish and human cell lines. Zebrafish were used to determine the ability of rs1967017 to direct tissue-specific gene expression, and luciferase assays in HEK293 and HepG2 cells were used to detect the effect of rs1967017 on transcription amplitude.
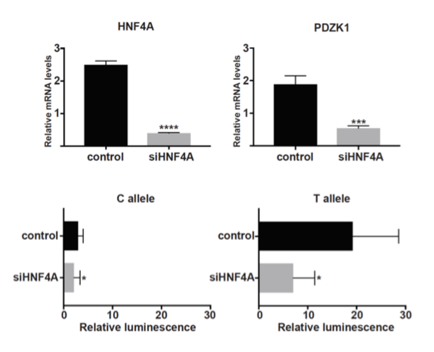
Results: PAINTOR analysis revealed rs1967017 to be the most likely causal variant in the region (Posterior Prob=0.93). Rs1967017 is a cis-acting eQTL for PDZK1 in the colon and small intestine with a high probability for the association signal with urate levels and the association signal with PDZK1 to be the same (COLOC Posterior Prob=0.93), meaning that rs1967017 likely influences urate levels through an influence on PDZK1 expression. The region harboring rs1967017 was capable of directly driving green fluorescent protein expression in the kidney and intestine of zebrafish embryos, indicating a conserved ability to confer tissue-specific expression. The urate-increasing T-allele of rs1967017 introduces a binding site for the transcription factor HNF4A. siRNA depletion of HNF4A reduced endogenous PDZK1 expression in HepG2 cells. Luciferase assays showed that the T-allele of rs1967017 gains enhancer activity relative to the C-allele, with T-allele enhancer activity abrogated by HNF4A depletion (Figure).
Conclusion: Our data predict that the urate-raising T-allele of rs1967017 enhances HNF4A binding to the PDZK1 promoter, thereby increasing PDZK1 expression. Because PDZK1 is a scaffold protein for uric acid transporters, its increased expression may contribute to reduced excretion of uric acid.
To cite this abstract in AMA style:
Merriman TR, Ketharnathan S, Boocock J, Phipps-Green A, Antony J, Leaask M, O'Sullivan J, Horsfield J. A Non-Coding Genetic Variant Maximally Associated with Serum Urate Levels Is Functionally Linked to HNF4A-Dependent PDZK1 Expression [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/a-non-coding-genetic-variant-maximally-associated-with-serum-urate-levels-is-functionally-linked-to-hnf4a-dependent-pdzk1-expression/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-non-coding-genetic-variant-maximally-associated-with-serum-urate-levels-is-functionally-linked-to-hnf4a-dependent-pdzk1-expression/