Session Information
Date: Saturday, November 16, 2024
Title: Plenary I
Session Type: Plenary Session
Session Time: 9:15AM-10:45AM
Background/Purpose: Use of low-dose glucocorticoids to maintain remission in patients with granulomatosis with polyangiitis (GPA) remains controversial. Additionally, there is no consensus on how to taper glucocorticoids in the late phases of clinical trials of GPA. The Assessment of Prednisone In Remission (TAPIR) trial aimed to evaluate the efficacy and safety of low-dose glucocorticoids for maintenance of remission of GPA.
Methods: Patients with GPA were eligible for TAPIR if they were i) within one year of treatment to induce remission for active disease; ii) in remission (BVAS/WG=0); and iii) receiving prednisone 5-20 mg/day. After tapering to prednisone 5 mg/day patients were randomized to either remain on prednisone 5 mg/day until Month 6 (M6) or taper off prednisone to 0 mg/day in 4 weeks and remain off glucocorticoids until M6. Other immunosuppressive therapy was continued. The primary outcome (M6) was rate of relapse, defined as a physician decision to increase the dose of glucocorticoids to treat GPA. Secondary outcomes included safety, patient-reported outcomes (PROs: PROMIS domains, SF-36), duration of remission, and severity of relapses. Patients were enrolled at 9 sites in US/Canada, or through an online system. Relapse rates were compared using logistic regression. Changes in PROs from baseline to M6 were compared by linear mixed effects models. Times to first relapse were compared by log-rank test.
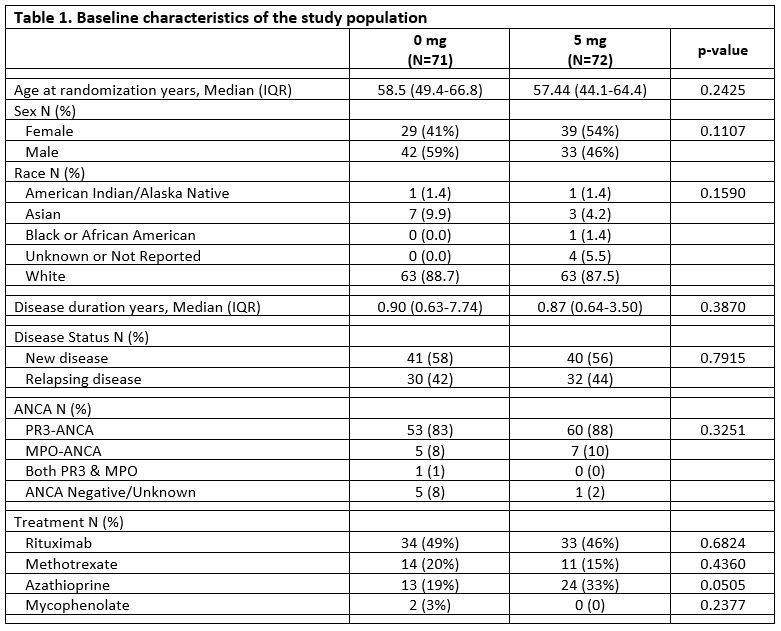
Results: 143 evaluable patients with GPA were randomized to the 5 mg/day prednisone group (N=72) or the 0 mg/day prednisone group (N=71). There were no differences in the baseline demographic and disease characteristics between arms (Table 1). Rituximab was prescribed for 54% of patients during the trial.
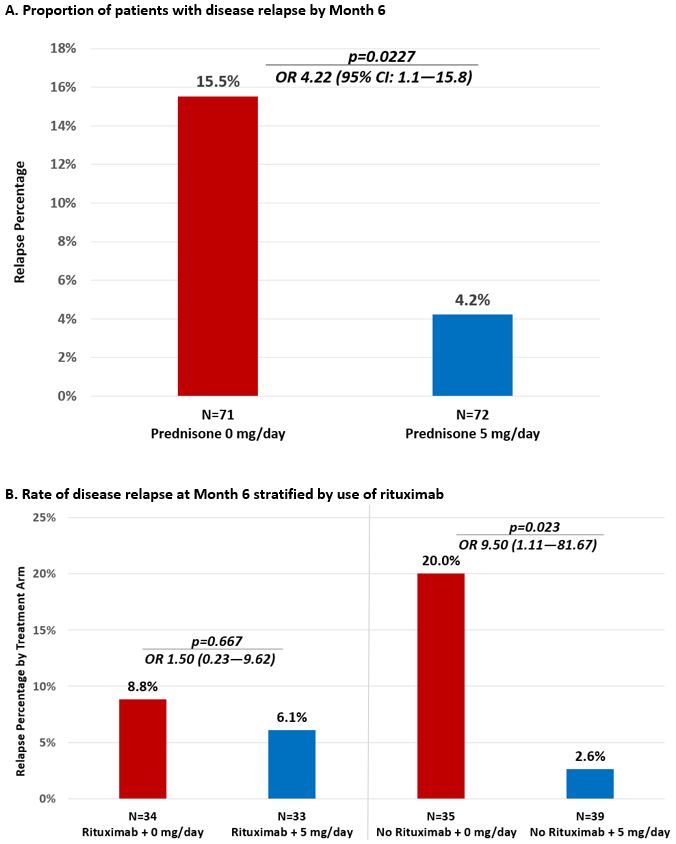
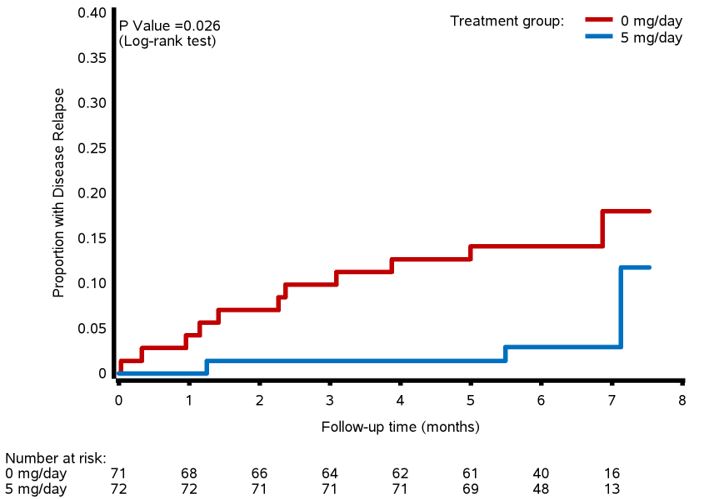
For the primary outcome of relapse by Month 6, 11/71 (15.5%) patients in the 0 mg arm experienced a relapse of GPA vs. 3/73 (4.2%) patients in the 5 mg arm, OR 4.22 (95% CI: 1.1-15.8), Figure 1A. When stratified by use of rituximab, there was no difference in the proportion with relapse at M6 for patients taking rituximab [0mg vs 5mg:8.8% vs. 6.1%, OR 1.50 (CI: 0.23-9.62), p=0.667] but for patients not taking rituximab, those in the 0 mg arm relapsed significantly more often than patients in the 5 mg arm [20.0% vs. 2.6%, OR 9.50 (CI 1.11-81.67), p=0.023]. Time to relapse was significantly shorter in the 0 mg arm vs. the 5 mg arm (p=0.026, Figure 2). All but one relapse during the trial were minor (93%). There were no differences between arms in change from Baseline to M6 for PROs [PROMIS Fatigue, Pain Interference, Physical Function; SF-36]. 6 serious adverse events occurred in 5 patients: 5 in the 0 mg arm and 1 in the 5 mg arm p=0.492. 10 infections occurred in the 0 mg arm and 4 in the 5 mg arm, p=0.555.
Conclusion: In GPA, after induction of remission, use of low-dose prednisone (5 mg/day) prevents more disease relapses over 6 months than being fully off prednisone (0 mg/day), but does not impact PROs. With either dose of prednisone the risk of a major relapse is extremely low. The benefit of low-dose prednisone (5 mg/day) to prevent minor flares is seen only among patients treated with a non-rituximab-based regimen. These results have important implications for clinical practice and clinical trial design.
To cite this abstract in AMA style:
Merkel P, Pagnoux C, Khalidi N, Specks U, Koening C, Langford C, Moreland L, Monach P, Springer J, Banerjee S, Carette S, Rhee R, Soowamber M, Warrington K, Borchin R, Burroughs C, McAlear C, Cuthbertson D, Krischer J. A Multicenter, Randomized, Controlled Trial to Evaluate the Effects of Low-dose Glucocorticoids Compared to Stopping Glucocorticoids to Maintain Remission of Granulomatosis with Polyangiitis: The TAPIR Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-multicenter-randomized-controlled-trial-to-evaluate-the-effects-of-low-dose-glucocorticoids-compared-to-stopping-glucocorticoids-to-maintain-remission-of-granulomatosis-with-polyangiitis-the-tapi/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-multicenter-randomized-controlled-trial-to-evaluate-the-effects-of-low-dose-glucocorticoids-compared-to-stopping-glucocorticoids-to-maintain-remission-of-granulomatosis-with-polyangiitis-the-tapi/