Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
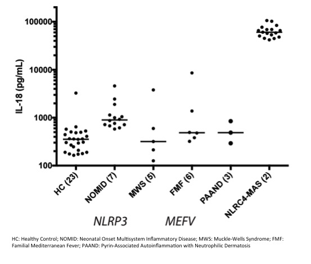
Background/Purpose: Patients prone to the development of Macrophage Activation Syndrome (MAS) can have extreme and often chronic elevation in the pro-inflammatory cytokine interleukin-18 (IL-18). In particular, those with gain-of-function mutations in the inflammasome-nucleating protein NLRC4 provided the first mechanistic link between the inflammasome, IL-18, and MAS. Other inflammasome-associated disorders lack such extreme and persistent IL-18 elevation (Fig. 1). In order to dissect the mechanisms associated with NLRC4 hyperactivity, we generated a mouse with an MAS-associated germline missense mutation (T337S) in Nlrc4 (N4-TS mice).
Methods: N4-TS mice were generated by Crispr-Cas9 genome engineering and bred to WT for at least four generations. IL-18 was measured by bead-based immunoassay (Luminex or BD Cytometric Bead Array). RNA-sequencing was performed on duodenal epithelial mucosal tissue scrapings placed into Trizol. MHC-II upregulation was assessed by flow cytometry.
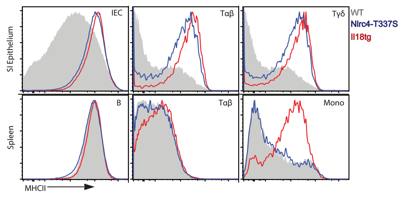
Results: N4-TS mice overproduced IL-18 in an allele-dependent manner. We found that IL-18 elevation was present as early as 3 weeks of age, and was unaffected by co-housing or antibiotic treatment. Using bone-marrow chimeras, we determined that IL-18 elevation in N4-TS mice was non-hematopoietic. Upon review of publicly available transcriptional datasets, we discovered that barrier epithelia, and in particular intestinal epithelial cells (IECs), are a rich source of colonization-independent Il18 and expressed high levels of Nlrc4 compared to other inflammasome nucleators. RNA-seq of duodenal epithelium showed upregulation of pathways associated with antigen presentation and epithelial turnover in N4-TS mice. Though we saw no increase in Dextran Sodium Sulfate-Induced colitis in N4-TS mice, increased baseline IEC proliferation was confirmed by EdU incorporation. MHC-II was upregulated in N4-TS intestinal epithelial cells as well intraepithelial and αβ- and γd-T-cells, but not in splenic or liver lymphocytes. By contrast, mice with transgenic expression of Il18 (Il18tg) had MHC-II upregulation in intestines, liver, and spleen. (Fig. 2)
Conclusion: Like NLRC4-MAS patients, N4-TS mice spontaneously overproduce IL-18. Barrier epithelia may be an important site for NLRC4-dependent IL-18 maturation. MHC-II upregulation is tissue-specific in Nlrc4-T337S mice and may be an anti-inflammatory response. These data suggest a role for barrier epithelial dysfunction as a contributor to systemic inflammatory diseases, particularly those associated with chronic IL-18 elevation and MAS.
To cite this abstract in AMA style:
Weiss E, Schneider C, Canna S. A Germline Macrophage Activation Syndrome-Associated Nlrc4 Mutation Causes Chronic, Systemic, Non-Hematopoietic IL-18 Elevation and Intestinal MHC-II Upregulation [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/a-germline-macrophage-activation-syndrome-associated-nlrc4-mutation-causes-chronic-systemic-non-hematopoietic-il-18-elevation-and-intestinal-mhc-ii-upregulation/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-germline-macrophage-activation-syndrome-associated-nlrc4-mutation-causes-chronic-systemic-non-hematopoietic-il-18-elevation-and-intestinal-mhc-ii-upregulation/