Session Information
Date: Wednesday, October 24, 2018
Title: 6W007 ACR Abstract: Patient Outcomes, Preferences, & Attitudes II: PROs (2910–2915)
Session Type: ACR Concurrent Abstract Session
Session Time: 9:00AM-10:30AM
A Draft Modified Core Domain Set for Patient-Reported Outcomes (PRO) in Patients with Idiopathic Inflammatory Myopathies (IIM): An OMERACT Report
Background/Purpose: The OMERACT Myositis special interest group (SIG) represents clinicians, patients, and researchers from four continents. Focus groups were conducted including 61 patients on three countries resulting in a list of 26 domains (1). In collaboration with International Myositis Assessment Clinical Study Group (IMACS), our goal was to identify a set of core patient-reported outcomes (PRO) in regards to life impact important to assess in clinical trials and clinical practice in myositis.
Methods: Patients with adult polymyositis, dermatomyositis, antisynthetase syndrome, or immune-mediated necrotizing myopathy (IMNM) in South Korea, Sweden and USA (N=638) responded to the first online modified Delphi in 2016. The second modified Delphi included patients (N=563), health care providers (HCP) (N=101), care givers (N=27) and regulatory agencies (n=xx) from multiple countries in 2017. A third modified Delphi was administered in 2018 including 410 patients, 109 HCP, 22 caregivers.
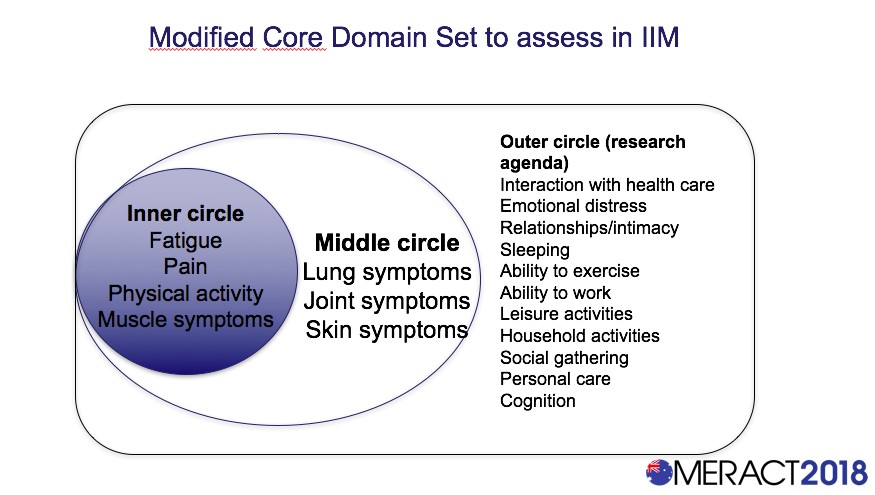
Results: From this work, four domains were deemed mandatory to measure in all clinical trials for IIM: fatigue, pain, levels of physical activity, and muscle symptoms (Figure 1). Additional optional domains include skin symptoms, lung symptoms, and joint symptoms. Several other domains were deemed important to study with further research efforts including sleeping difficulty, cognitive distress, ability to work, and emotional distress.
Conclusion: A draft set of core PRO has been developed through validated methods based on OMERACT guidelines. Fatigue, pain, levels of physical activity and muscle symptoms were included in the inner circle and should always be used in clinical trials in IIM. We next seek to develop corresponding instruments with each of these domains with future efforts.
Reference: Regardt M et al. Patients’ experience of myositis and further validation of a patient-reported outcome measure – establishing core domains and expanding patient input on clinical assessment in myositis. Report from OMERACT 12. J Rheumatol 2015;42:2492-5.
Figure 1: Draft Modified Core Domain Set for PROs in IIM Patients
To cite this abstract in AMA style:
Regardt M, Mecoli CA, Park JK, Needham M, De Groot I, Sarver C, Lundberg IE, Shea B, De Visser M, Song YW, Bingham III CO, Christopher-Stine L, Alexanderson H. A Draft Modified Core Domain Set for Patient-Reported Outcomes (PRO) in Patients with Idiopathic Inflammatory Myopathies (IIM): An Omeract Report [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/a-draft-modified-core-domain-set-for-patient-reported-outcomes-pro-in-patients-with-idiopathic-inflammatory-myopathies-iim-an-omeract-report/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-draft-modified-core-domain-set-for-patient-reported-outcomes-pro-in-patients-with-idiopathic-inflammatory-myopathies-iim-an-omeract-report/