Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Diagnostic delays are common with psoriatic arthritis (PsA) and contribute to unnecessary impairments in quality of life and function. Screening surveys for identifying at-risk patients are infrequently used in busy clinical practices. The goal was to develop a direct-to-patient screening approach that does not burden providers with PsA screening tasks. Objective 1 was to characterize patterns of rheumatology referrals and PsA diagnoses in psoriasis patients randomized to receive a screening survey (intervention group) vs. not receive a survey (control group). Objective 2 was to characterize referral and diagnostic patterns in subgroups of intervention group patients instructed to either contact study staff for a rheumatology appointment (direct access [DA] group) or discuss a rheumatology referral with their doctor (standard of care [SOC] group), if they screened positively for elevated PsA risk.
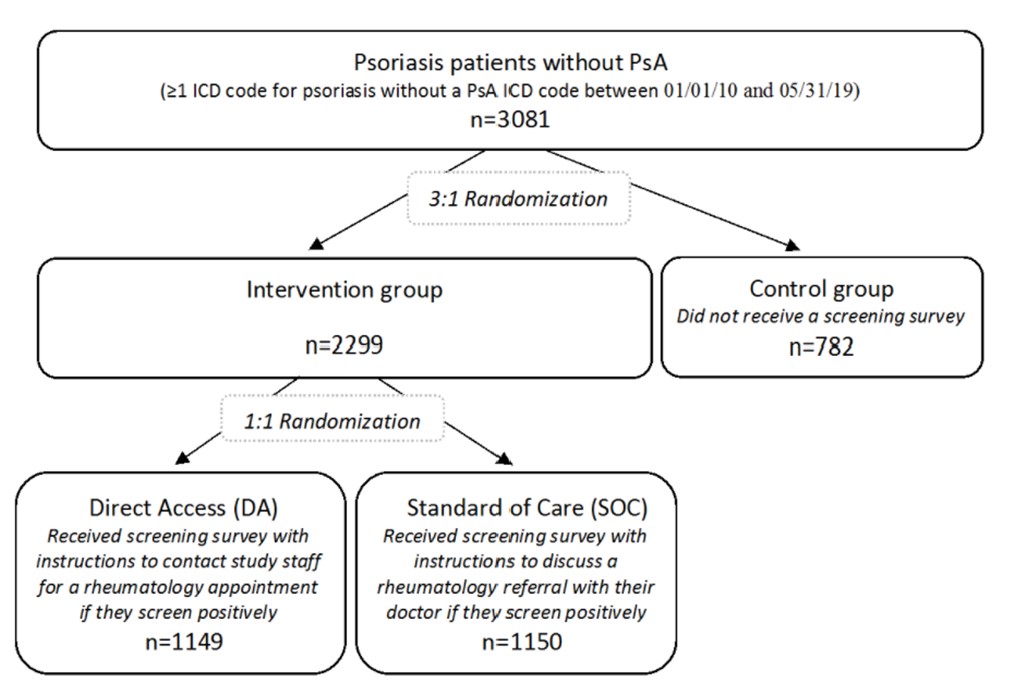
Methods: Patients with an International Classification of Diseases (ICD) code for psoriasis but not PsA in the electronic health record (EHR) were randomized to the control group vs. intervention groups (DA or SOC) (Figure 1). The Psoriasis Epidemiology Screening Tool Survey1 was disseminated electronically and via postal mail beginning 09/18/20 (index date). Referral and diagnostic outcomes were extracted from the EHR for preliminary analysis on 2/2/2021 and compared between DA, SOC, and control groups.
Results: The population included 3081 patients (Figure 1) with 51.5% females and a mean age of 50.1 years. 15.4% completed the online survey, of whom 29.3% screened positively. Among DA patients, 8.6% contacted study staff, of whom 32.5% screened positively, and 29.3% requested a rheumatology appointment. In EHR data, rheumatology appointments occurred with 1.4% DA, 0.7% SOC, and 0.1% control patients, and the mean times to a rheumatology appointment were 77.4, 60.6, and 133.0 days in DA, SOC, and control groups, respectively (Figure 2). PsA diagnosis occurred in 0.4% DA, 0.3% SOC, and 0 control patients, and mean times to PsA diagnosis were 66.0 days in DA and 77.0 days in SOC groups.
Conclusion: Rheumatologic evaluations and PsA diagnoses were more frequent and earlier in patients exposed to a screening survey than unexposed patients. Rates of rheumatologic evaluations and PsA diagnosis were low, but patient engagement may improve with future study cohorts (n≈24,000 patients), as the impacts of the COVID-19 pandemic decline.
1Ibrahim GH, et al. Clin Exp Rheumatol. 2009; 27:469-74.
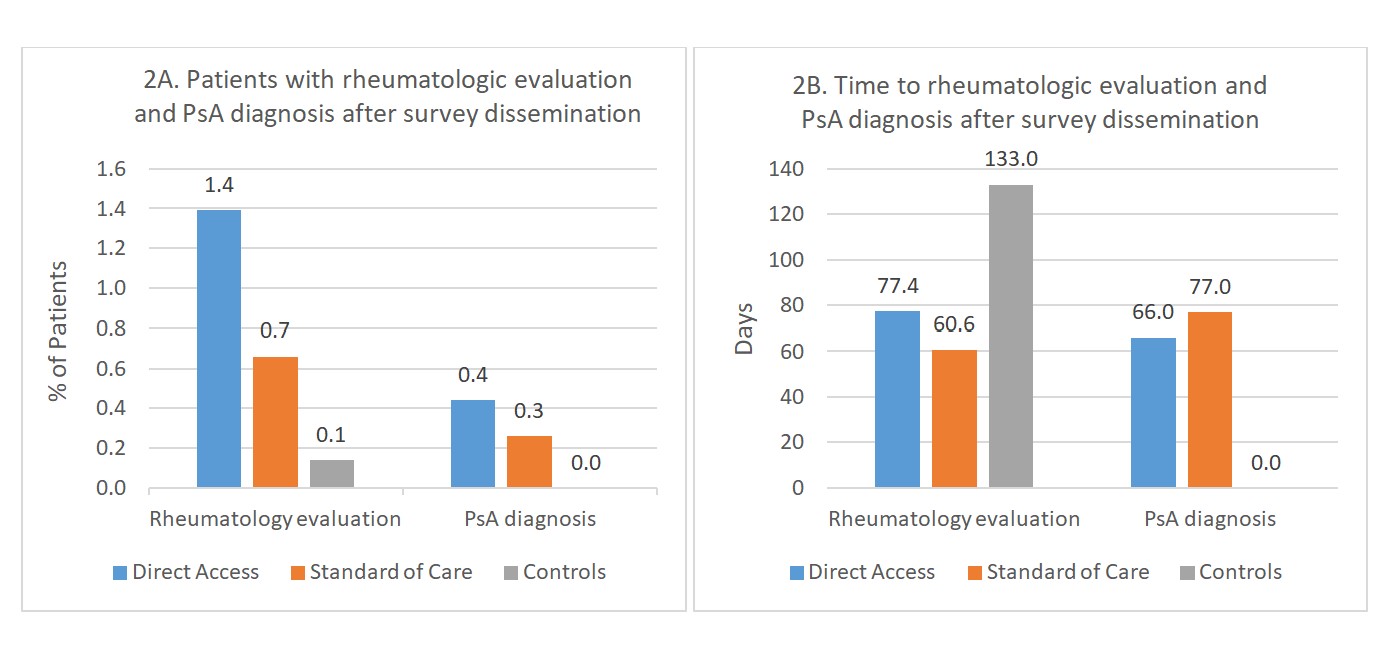 Figure 2. Referral and diagnostic patterns for PsA in patients with psoriasis
Figure 2. Referral and diagnostic patterns for PsA in patients with psoriasis
To cite this abstract in AMA style:
Meier M, Walsh J, Pei S. A Direct-to-Patient PsA Screening Survey for Earlier Identification of At-Risk Psoriasis Patients and Reduction of Physician Burden [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/a-direct-to-patient-psa-screening-survey-for-earlier-identification-of-at-risk-psoriasis-patients-and-reduction-of-physician-burden/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-direct-to-patient-psa-screening-survey-for-earlier-identification-of-at-risk-psoriasis-patients-and-reduction-of-physician-burden/