Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The availability of published long-term data from systemic lupus erythematosus (SLE) clinical trials is limited [1]. To complement existing belimumab data, we examined the long-term safety and efficacy of belimumab plus standard SLE therapy (SoC) in patients who participated in the open-label extension that followed BLISS-76.
Methods: This was a multicenter, continuation study (GSK study 112233; NCT00724867) of patients who completed BLISS-76 in the US. In this study patients received the same dose of belimumab as in BLISS-76 (1 or 10 mg/kg IV, every 28 days) plus SoC; patients who had previously received placebo received belimumab 10 mg/kg IV. Following licensing of belimumab, the dose for patients who received 1 mg/kg was increased to 10 mg/kg. Primary outcome measures included long-term safety, assessed by adverse event (AE) frequency and Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI) evaluated every 48 weeks (Study Year). Other assessments included SLE Responder Index (SRI), prednisone use, B cell levels, and flare rates (modified SLE Flare Index [SFI]).
Results: 268 patients comprised the modified intent-to-treat (MITT) population; 140 patients completed the continuation study (52.2%), and 128 (47.8%) withdrew (patient request, 24.2%; AE, 19.5%). At baseline, the mean (SD) Safety of Estrogens in Lupus Erythematosus National Assessment SLE Disease Activity Index (SELENA-SLEDAI) score was 7.8 (3.86), and the mean (SD) SDI score was 1.2 (1.51). The incidence of overall AEs, treatment-related AEs, and serious AEs remained stable or declined through Study Year 7 (Table). The mean (SD) SDI score increased by 0.4 (0.68) from baseline to Study Year 7 (MITT population). 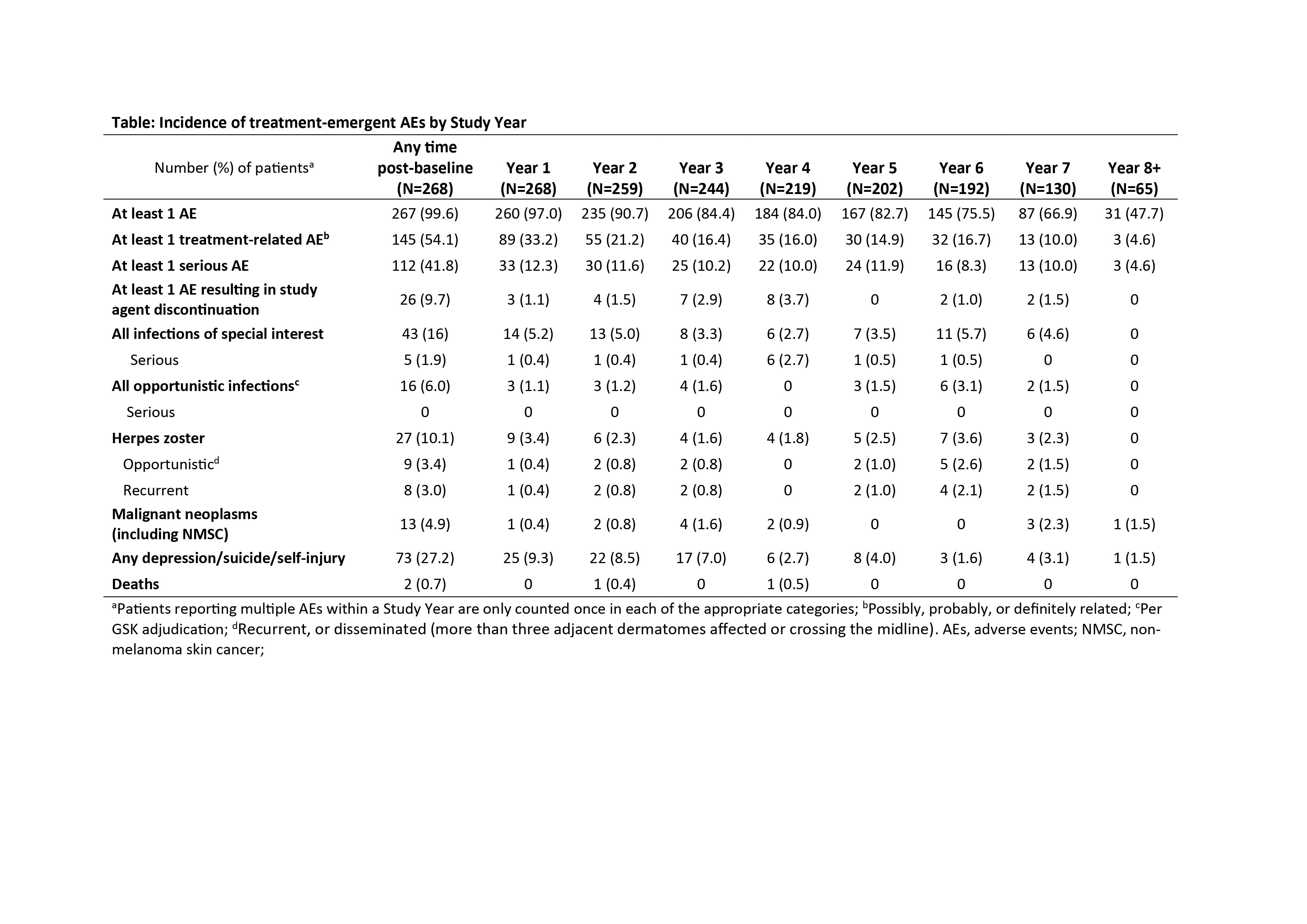
An SRI response was achieved by 41.9% (96/229) of patients overall at Study Year 1 Midpoint (Week 24), increasing to 75.6% (90/119) at Study Year 7 Midpoint. At Study Year 7 Midpoint, relative to baseline, 78.2% (93/119) of patients overall achieved a ≥4-point SELENA-SLEDAI score reduction, 98.4% (125/127) had no new British Isles Lupus Assessment Group (BILAG) 1A/2B organ domain scores, and 93.7% (119/127) of patients had no worsening in Physician’s Global Assessment (PGA). The median decrease in prednisone dose from baseline was 47.1% (n=77, those receiving prednisone), and the median change in CD20+ B cells was -83.2%, at Study Year 7 Midpoint. Severe SFI flare was experienced by 20.6% (55/267) of patients through Study Year 7 Midpoint.
Conclusion: Long-term exposure to belimumab was generally safe and well tolerated. The incidence of AEs remained stable or declined throughout the study. These results are consistent with data from the Phase 2 extension study and the known profile of belimumab in patients with SLE. Study funded by GSK/Human Genome Sciences, Inc. Nicole Cash, PhD, Fishawack Indicia Ltd, UK, provided editorial assistance, funded by GSK. 1. Ginzler EM, et al. J Rheumatol. 2014;41(2):300–9
To cite this abstract in AMA style:
Furie RA, Wallace DJ, Aranow C, Fettiplace J, Wilson B, Mistry P, Roth DA, Gordon D. 7-Year Safety and Efficacy of Belimumab in Patients with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/7-year-safety-and-efficacy-of-belimumab-in-patients-with-systemic-lupus-erythematosus/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/7-year-safety-and-efficacy-of-belimumab-in-patients-with-systemic-lupus-erythematosus/
