Session Information
Date: Monday, November 11, 2019
Title: 4M096: Spondyloarthritis Including Psoriatic Arthritis – Clinical III: Miscellaneous (1818–1823)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Secukinumab is a fully human IgG1 monoclonal antibody targeting interleukin-17A. There is a lack of real-life evidence on secukinumab retention rates and treatment outcomes in axial spondyloarthritis (axSpA) patients. Hence, the aim of this study was to determine the 6- and 12-month secukinumab retention rates as well as the crude and LUNDEX corrected proportions of patients in remission after 6 and 12 months of treatment in Europe. This was assessed overall as well as stratified by prior biologic disease-modifying anti-rheumatic drug (bDMARD)/targeted synthetic (ts)DMARD use.
Methods: Data from axSpA patients treated with secukinumab in routine care from 12 countries in the European Spondyloarthritis (EuroSpA) Research Collaboration Network were pooled. Time from treatment initiation to data cut was ≥ 12 months regardless of treatment durations and cover start date between May 2015 and April 2018. The following outcomes were calculated: Proportions of patients achieving Bath Ankylosing Spondylitis Disease Activity Score (BASDAI) < 2 / BASDAI < 4 and Ankylosing Spondylitis Disease Activity Score (ASDAS) < 1.3/ ASDAS < 2.1 at 6 and 12 months, including with LUNDEX1 adjustments. Group comparisons between b/tsDMARD naïve and 1 or ≥2 prior b/tsDMARD users were performed with ANOVA, Kruskal-Wallis or Chi-square test or with Kaplan-Meier analyses with log rank test, as appropriate.
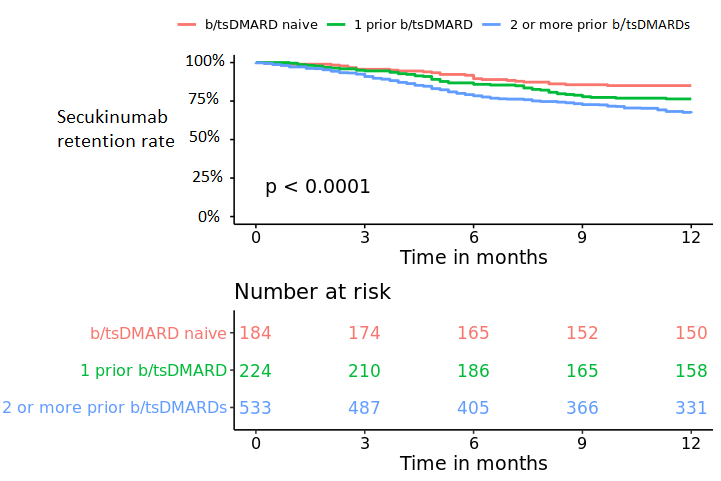
Results: A total of 941 axSpA patients were included, thereof 6 who started treatment in 2015, 215 in 2016, 573 in 2017 and 147 in 2018. Overall 6/12-month secukinumab retention rate was 82%/73% and higher in b/tsDMARD naïve compared to non-naïve patients (table, figure). After 6/12 months treatment BASDAI < 4 was achieved by 51%/62%, BASDAI < 2 by 24%/34%, ASDAS < 2.1 by 23/33% and ASDAS < 1.3 by 8%/14% of the patients. b/tsDMARD naïve patients compared with patients treated with 1 prior or 2 or more prior b/tsDMARDs had shorter time since diagnosis, higher baseline disease activity and a higher proportion were men. Overall, LUNDEX adjusted 6 and 12 months’ responses were achieved more often in b/tsDMARD naive patients than in patients who had received 1 or 2 or more previous b/tsDMARDs (table).
Conclusion: This study of >900 patients in 12 European countries provided real-world data on the effectiveness of secukinumab in patients with axSpA, adding evidence to existing RCTs. A majority of the patients were treated with 2 or more previous b/tsDMARDs and had long disease duration. Overall retention rate was 82%/73% at 6/12 months, respectively, with higher retention rates for b/tsDMARD naïve compared with patients treated with 1 or 2 or more previous b/tsDMARDs. Overall, a higher proportion of bionaïve than previous b/tsDMARD users achieved remission regardless of remission criteria.
References: 1Kristensen et al. Arthritis Rheum 2006, 54(2):600-606
To cite this abstract in AMA style:
Michelsen B, Askling J, Codreanu C, Mann H, Loft A, Pombo-Suarez M, Rotar Z, Kvien T, Santos M, Hokkanen A, Iannone F, Gudbjornsson B, Onen F, Jacobsson L, IONESCU R, Pavelka K, Sánchez-Piedra C, Tomsic M, Sexton J, Santos H, Österlund J, Cauli A, Geirsson A, Akar S, Ciurea A, Jones G, van der Horst-Bruinsma I, Heegaard Brahe C, Georgiadis S, Midtbøll Ørnbjerg L, Østergaard M, Lund Hetland M. 6 and 12-month Drug Retention Rates and Treatment Outcomes in 941 Patients with Axial Spondyloarthritis Treated with Secukinumab in Routine Clinical Practice in 12 European Countries in the EuroSpA Research Collaboration Network [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/6-and-12-month-drug-retention-rates-and-treatment-outcomes-in-941-patients-with-axial-spondyloarthritis-treated-with-secukinumab-in-routine-clinical-practice-in-12-european-countries-in-the-eurospa-re/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/6-and-12-month-drug-retention-rates-and-treatment-outcomes-in-941-patients-with-axial-spondyloarthritis-treated-with-secukinumab-in-routine-clinical-practice-in-12-european-countries-in-the-eurospa-re/