Session Information
Title: Systemic Lupus Erythematosus - Clinical Aspects and Treatment: Treatment and Management Studies
Session Type: Abstract Submissions (ACR)
Background/Purpose: The clinical efficacy of belimumab in patients with systemic lupus erythematosus (SLE) has been demonstrated in large randomized clinical trials. We examined clinical outcomes following belimumab treatment in clinical practice settings in the US.
Methods: OBSErve US (evaluation Of use of Belimumab in clinical practice SEttings in the US; GSK Study: BLM117295) was a multicenter, retrospective, medical chart review study. Rheumatologists from non-academic centers were randomly recruited from a national physician database. Physicians reported retrospective data from medical charts of randomly identified adult SLE patients in their care who had received ≥8 belimumab infusions as part of usual care. Data were reported for 6 months prior to index date (date of first belimumab infusion), and every 6 months thereafter for up to 24 months. The primary outcome measure was physician impression of change in SLE disease manifestations, relative to the previous time point. Here we report the final analyses of patients who had outcomes reported at Month 24.
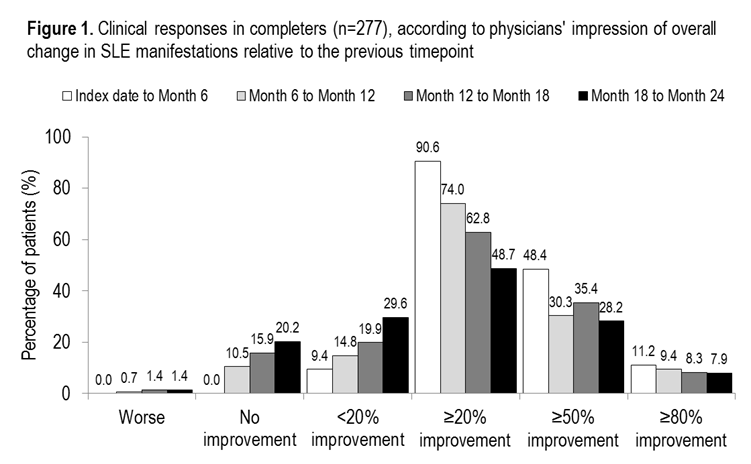
Results: At index date, 501 patient charts were analyzed. By Month 24, 112 patients were lost to follow-up and 112 patients had discontinued. Most common reasons for discontinuation included patient request (n=44, 40.2%), medication not effective (n=33, 29.5%), disease progression (n=15, 13.4%), loss of insurance or reimbursement (n=14, 12.5%) and lack of patient compliance (n=11, 9.8%). The Month 24 completer analysis included 277 patients: female, 90.6%; mean age, 42.9 (standard deviation [SD]: 12.0) years; Caucasian, 52.7%; African-American, 24.9%; Hispanic, 15.5%; Other, 6.9%.
Of the 277 patients, 134 (48.4%) had ≥50% improvement in overall clinical response between index date and Month 6 according to the physicians’ impression (Figure 1). Further improvements were observed during Months 6–12 and 12–18. At Month 24, 78 (28.2%) patients had improved by ≥50% since Month 18.
At index date, 218/277 (78.7%) patients received concomitant steroids (mean [SD] dose: 18.0 [12.2] mg/day); by Month 24, the mean (SD) dose among these patients was 2.9 (3.4) mg/day and 95 (43.6%) patients had discontinued steroids. During the 24-month period six patients initiated steroid treatment.
Of the 277 patients who completed to Month 24, 69 (24.9%) had SELENA-SLEDAI scores available at index date and Month 24; the mean (SD) score at index date was 12.5 (3.0), with a reduction of 8.2 (3.8), to 4.4 (3.1) by Month 24.
Conclusion: Overall, physicians reported continued improvements in clinical outcomes throughout the study, among patients who had completed 24 months of treatment with belimumab 10 mg/kg plus usual care (≥8 belimumab infusions). Over 24 months, mean steroid dose among baseline users was reduced by 84%.
Study funded by GSK and Human Genome Sciences, Inc., USA. Medical writing support provided by L Pettinger, Fishawack Indicia Ltd, UK, funded by GSK.
Disclosure:
C. E. Collins,
GlaoxSmithKline,
5,
GlaxoSmithKline,
8,
Abbvie,
8;
H. Kan,
GlaxoSmithKline,
3,
GlaxoSmithKline,
1;
M. Dall’era,
None;
C. Macahilig,
Medical Data Analytics,
9;
R. Pappu,
GlaxoSmithKline,
3,
GlaxoSmithKline,
1;
C. T. Molta,
GlaxoSmithKline,
3,
GlaxoSmithKline,
1;
V. Koscielny,
GlaxoSmithKline,
3,
GlaxoSmithKline,
1.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/24-month-outcomes-associated-with-belimumab-in-patients-with-systemic-lupus-erythematosus-in-clinical-practice-settings/