Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by diverse clinical manifestations and organ damage accrual. Black/African-American (B/AA) race is associated with higher SLE prevalence, disease activity, organ damage, and mortality than in Caucasian patients Although the efficacy and safety of belimumab (BEL) has been evaluated in adult patients of B/AA race with active SLE in a randomized clinical trial (EMBRACE; NCT01632241), real-world BEL effectiveness data in B/AA patients is limited. We report clinical outcomes in B/AA patients with SLE treated with BEL in a US clinical practice setting.
Methods: OBSErve US (evaluation Of use of Belimumab in clinical practice SEttings in the US; GSK Study 117295) was an observational cohort study that randomly recruited non-academic rheumatologists from a national physician database. Data were collected on randomly selected adult patients with SLE who had received ≥8 BEL infusions as part of standard care. Patient demographics, disease characteristics, clinical outcomes, healthcare resource utilization, and oral corticosteroid (OCS) use were extracted from medical charts for 6 months prior to index date (1st BEL infusion), and then prospectively every 6 months up to Month 24. The primary outcome was physician-assessed overall clinical response relative to the previous 6-month time point. Post hoc analysis was performed (GSK Study 208439) on the B/AA subpopulation who completed the 24-month study period.
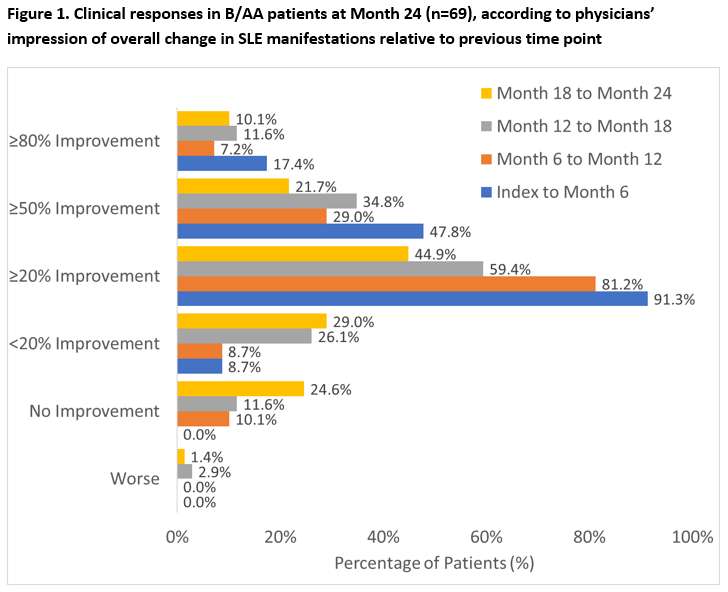
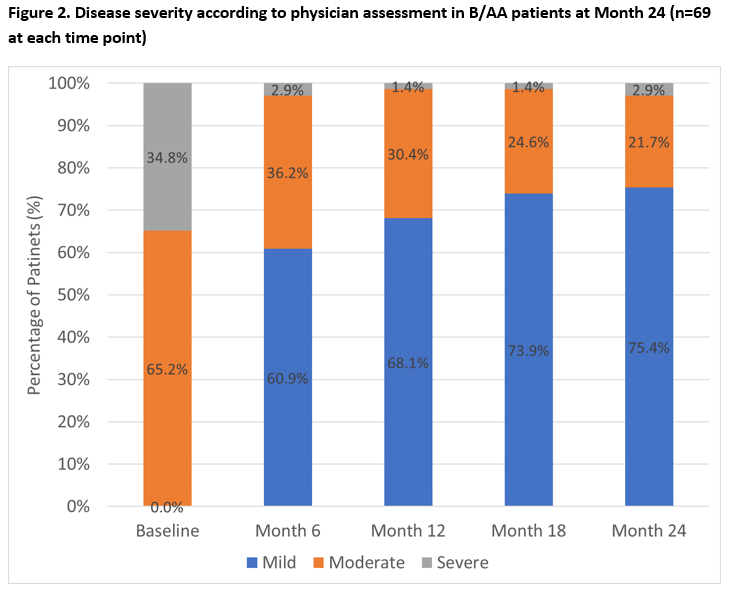
Results: OBSErve US enrolled 501 patients; 123 (24.5%) were B/AA and 69/123 (56.1%) B/AA patients continued to receive BEL at Month 24. Baseline (BL) characteristics of the Month 24 completers included: female 88.4%, mean age (standard deviation [SD]) 41.6 (12.5) years; severe SLE at BEL initiation (34.8%), and key clinical diagnoses: lupus nephritis (27.5%) and hypertension (26.1%). At Month 24, 33/69 (47.8%) B/AA patients had ≥50% improvement in physician-assessed overall clinical response between index and the previous 6 months, with further improvements observed in each subsequent 6-month period (Figure 1). By Month 24, 52/69 (75.4%) B/AA patients had mild SLE (severe SLE: 2.9%, Figure 2). Of the 69 B/AA patients with BL moderate/severe disease, 36/69 (52.2%) had consistent mild SLE at Month 6, 12, 18, and 24. At BEL initiation, 57/69 (82.6%) B/AA patients received OCS (mean [SD] dose: 19.7 [12.8] mg/day); and at Month 24, 40.4% had discontinued OCS with a mean (SD) OCS dose of 3.1 (3.2) mg/day.
Conclusion: OBSErve US results from the B/AA SLE subpopulation who received BEL over 24 months in a clinical practice setting suggested substantial improvements in clinical outcomes (eg, reduced disease activity, severity, OCS utilization). While results may be limited with regards to sample size, subjective assessments, and analysis-by-responder bias, they demonstrated the effectiveness and durability of BEL in B/AA patients with SLE over 24 months. Results might inform decision-making when considering BEL for the treatment of B/AA patients with SLE, particularly in the context of published BEL efficacy and data from clinical trials.
Study Funding: GSK.
To cite this abstract in AMA style:
Collins C, Kerr G, Von Feldt J, Rubin B, Katz A, Castellano V, Chung J, Bell C. 24-Month Outcomes Associated with Belimumab in Black/African-American Patients with Systemic Lupus Erythematosus in a Clinical Practice Setting in the United States [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/24-month-outcomes-associated-with-belimumab-in-black-african-american-patients-with-systemic-lupus-erythematosus-in-a-clinical-practice-setting-in-the-united-states/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/24-month-outcomes-associated-with-belimumab-in-black-african-american-patients-with-systemic-lupus-erythematosus-in-a-clinical-practice-setting-in-the-united-states/