Session Information
Date: Monday, November 13, 2023
Title: Abstracts: Spondyloarthritis Including Psoriatic Arthritis – Treatment II: PsA
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Oligoarticular PsA can be associated with significant impact on quality of life, despite limited joint involvement. The phase 4 FOREMOST study evaluated the efficacy of apremilast (APR) in patients (pts) with limited joint involvement (defined as 2–4 swollen and 2–4 tender joints [2–8 active joints]) using a modified minimal disease activity score (MDA-Joints).
Methods: FOREMOST (NCT03747939) is a phase 4, multicenter, randomized, double-blind, placebo (PBO)-controlled, parallel-group study. Eligible pts had early disease (PsA duration ≤5 years) and limited joint involvement ( >1 but ≤4 swollen and >1 but ≤4 tender joint count [SJC and TJC] of 66–68 joints assessed). Joints affected at baseline (BL) were defined as sentinel joints. Pts were randomized 2:1 to APR or PBO for 24 weeks, with an early escape at Week 16. The primary endpoint was the proportion of pts at Week 16 who achieved MDA-Joints (mandating SJC ≤1 and TJC ≤1 and 3/5 alternate items). Secondary endpoints assessed at Week 16 included the proportion of pts achieving Clinical Disease Activity in Psoriatic Arthritis (cDAPSA) remission (REM, ≤4) or low disease activity (LDA, >4 to ≤13), Patient’s Global Assessment of Disease Activity (PtGA) ≤20, patient assessment of pain ≤15, Psoriatic Arthritis Disease Activity Score (PASDAS) good or moderate response, and change from BL in Psoriatic Arthritis Impact of Disease 12-item (PsAID-12). Exploratory analyses were performed for all joints and posthoc analyses were conducted in pts with 2–4 sentinel joints. The proportions of pts with SJC or TJC >4 over time were also assessed by pts with a BL joint count of 2–4.
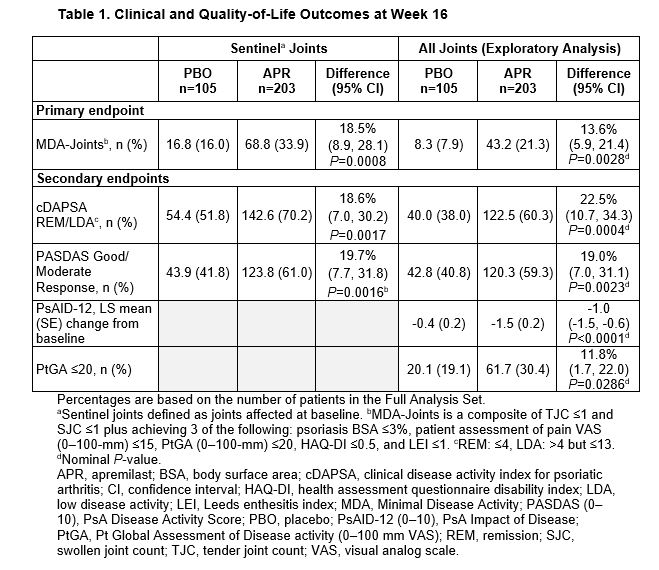
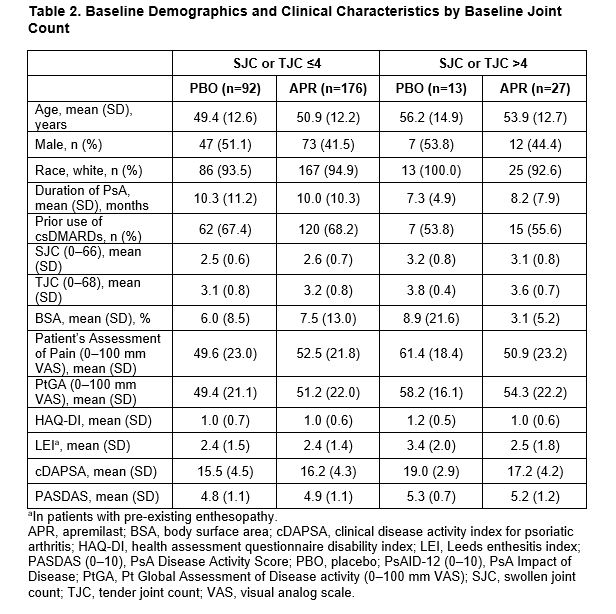
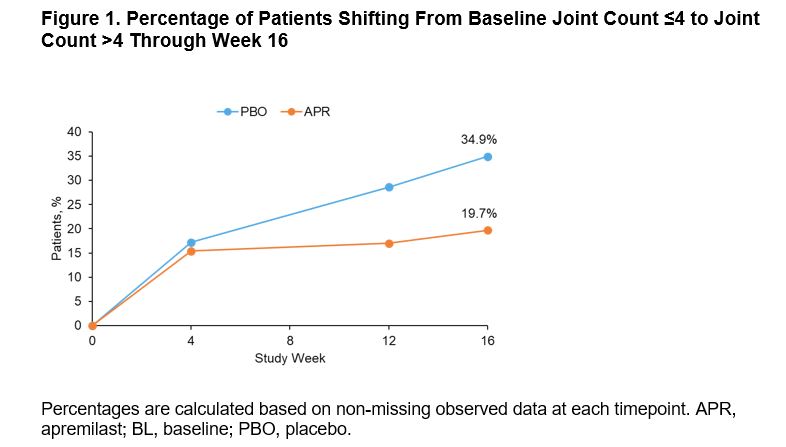
Results: Of 308 pts randomized (APR: n=203; PBO: n=105), mean PsA duration was 9.9 (SD 10.2) months, mean age was 50.9 (SD 12.5) years, and 39.9% of pts were using a csDMARD. In the overall population, MDA-Joints response (primary endpoint, based on sentinel joints) was achieved by significantly more pts with APR (33.9%) vs PBO (16.0%) at Week 16 (P=0.0008) (Table 1). Additionally, significantly greater proportions of pts achieved secondary endpoints with APR vs PBO at Week 16 (Table 1). Clinical characteristics were similar between pts with ≤4 joints and >4 joints involved at BL (Table 2). A total of 268 (87%) patients had ≤4 active joints at BL. In a post hoc analysis, similar MDA-Joints response rates were seen in pts with 2–4 joints (APR: 34.4%, PBO: 17.2%) vs the overall study population at Week 16. In pts with 2–4 joints involved at BL, there was an increase in the proportions of pts who switched to a joint count >4 through Week 16 among those receiving PBO but not among those receiving APR (Figure 1). No new safety signals were identified.
Conclusion: FOREMOST is the first global randomized controlled trial studying early oligoarticular PsA. In this study, better disease control is achievable with APR, with twice the MDA-Joint response compared with PBO at 16 weeks. A higher percentage of pts with BL joint count ≤4 shifted to a joint count of >4 with PBO vs APR.
To cite this abstract in AMA style:
Mease P, Gladman D, Coates L, Aelion J, Vasandani J, Kavanaugh A, Merola J, Reddy J, Wang R, Brunori M, Colgan S, Gossec L. 16-Week Results from FOREMOST, a Placebo-Controlled Study Involving Oligoarticular Psoriatic Arthritis Treated with Apremilast [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/16-week-results-from-foremost-a-placebo-controlled-study-involving-oligoarticular-psoriatic-arthritis-treated-with-apremilast/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/16-week-results-from-foremost-a-placebo-controlled-study-involving-oligoarticular-psoriatic-arthritis-treated-with-apremilast/