Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: A detailed understanding of fibromyalgia and its symptoms in the US population would be valuable. The National Health Interview Survey (NHIS) is the principal source of civilian population data in the USA. To study fibromyalgia in the NHIS requires the development of a fibromyalgia definition that uses NHIS questions and a subsequent validation of that definition with a standard fibromyalgia definition. However, the 2011 modified American College of Rheumatology (ACR) criteria and the NHIS questionnaire set evaluate pain differently and over different time periods. In particular, NHIS inquires about joints while the ACR criteria about painful regions. To reconcile and adjust for these differences we administered the ACR criteria and questions from the NHIS at the same time to rheumatic disease patients.
Methods: In 2012, NHIS collected data about joint pain, fatigue, subjective cognitive impairment, mood, headaches, and sleep. A questionnaire was developed that incorporated 2012 NHIS questions along with the questions of the 2011 modified ACR research criteria for fibromyalgia. These questionnaires were sequentially administered to 415 patients during office visits in 2 rheumatology clinics.
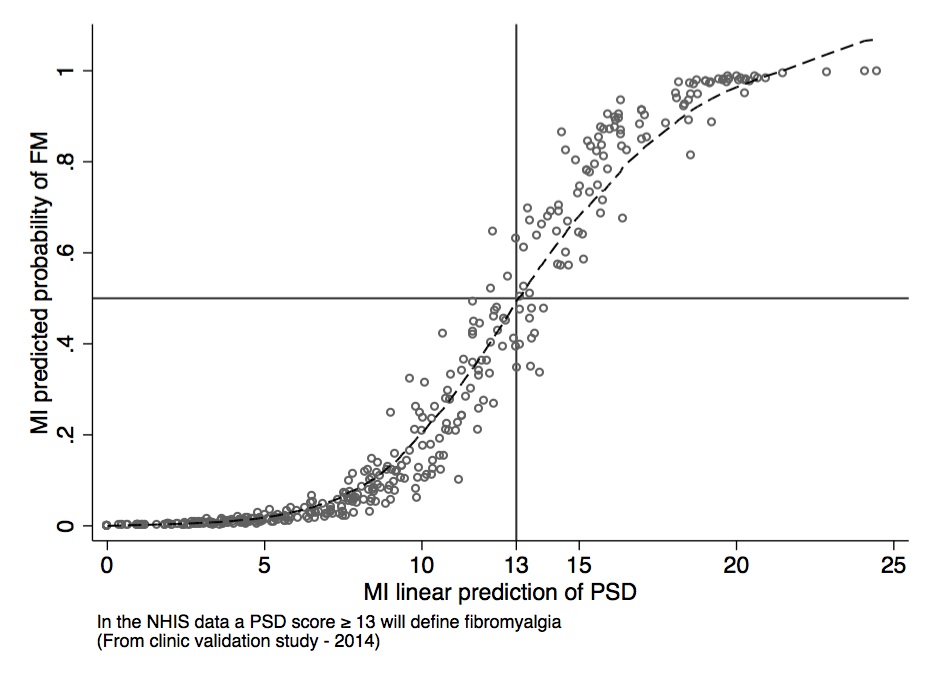
Results: For the 415 questionnaires, the average missing items per questionnaire was 1.4, with percent missing for items ranging between 0 and 19.2%. 274 questionnaires were completed without missing data. We used multiple imputation by chained equations and 10 iterations to develop a complete imputed set of 415 questionnaires. We tested a series of matching and regression techniques to identify the optimum set of predictors based on measurements of PSD and ACR criteria as determined by the 2010 modified ACR research criteria. The R-Squared for measured and predicted PSD was 0.781. In a logistic model, we tested NHIS predictors against observed ACR criteria positivity. The area under the receiver-operating curve (AUC ROC) was 94.6. 88.0% of cases were properly classified, and the sensitivity/specificity was 74.7%/93.4%.The NHIS definition found to best approximate 2010 ACR criteria included specific multiple painful joint sites, problems with concentration, fatigue and abdominal pain.
Conclusion: It is feasible to use NHIS questions to approximate both fibromyalgia diagnoses and polysymptomatic distress in 2012 NHIS data. This definition can be used for the epidemiologic study of fibromyalgia in NHIS.
Disclosure:
B. Walitt,
None;
R. Nahin,
None;
R. S. Katz,
None;
M. J. Bergman,
None;
F. Wolfe,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-fibromyalgia-and-polysymptomatic-distress-definitions-in-the-national-health-interview-survey/