Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Biologics are used commonly for patient with autoimmune diseases. These agents have ensured important efficacy advantages in the treatment of inflammatory rheumatic diseases. All biologics have been associated with risk of infections. The risk of serious infections has been reported to increase 1-2-1.8 times for patients on tumor necrosis factor inhibitors (TNFi) compared to patients on conventional agents. The risk of tuberculosis (TB) has been reported to increase manifold, up to 12 to 35 times. American College of Rheumatology (ACR) recommends that all patients on biologics should be screened for risk of latent TB infection (LTBI) either with tuberculin skin test (TST) or interferon-release assays (IGRAs), regardless of risk factors for LTBI.
With this Quality improvement exercise, we aim to improve compliance with TB screening in patients receiving biologics to a target of 90% over 3 months.
Methods:
We generated an EMR query to review pre-intervention screening rates of patients on biologics from 3 university clinics over 6 months (July-December 2013) . A list of 104 such patients was created. Chart review for these 104 patients was performed to determine adequacy of TB screening defined by presence of a documented TB screening, either PPD or Quantiferon gold TB test, within 1 year of last clinic visit. At baseline, TB screening had been performed in 78% of patients. To improve compliance, as an intervention, we conducted conferences with providers to educate them on the guidelines and our current performance. Nurses were educated through emails. Monthly email follow-up reminders were also sent to the providers and nurses. At 3 months a follow up chart review was conducted for 152 patients in the same three clinics, who had received prescriptions of biologics between March-June 2014 to determine effect of provider education on compliance with TB screening.
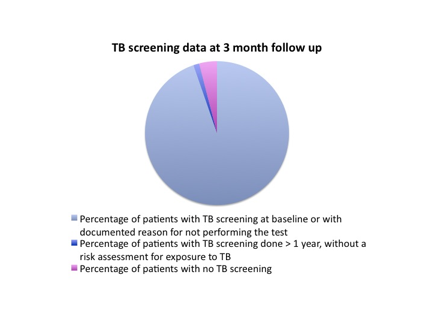
Results:
Educational conferences and emails were well received by providers and nurses. At follow up after 3 months, the target was achieved in all clinics, with compliance rates for TB screening at 94.7% across board.
Conclusion:
Developing methods to improve quality will become even more relevant in future. In this study, we demonstrated provider education makes a difference in implementation of guidelines for TB screening in patients receiving biologics. This was achieved in relatively short period of time. A similar strategy can be implemented in other subspecialty clinics like dermatology and gastroenterology where biologics are also being used.
Disclosure:
S. Jatwani,
None;
R. Rudrangi,
None;
K. Jatwani,
None;
V. Murthy,
None;
R. Maganti,
None;
R. McCallum,
None;
E. Gonzalez,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-compliance-for-tuberculosis-screening-for-patients-on-biologics-in-rheumatology-clinics/