Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Biologic medications have revolutionized the treatment of inflammatory arthritis. Subsequent entry biologics (SEBs) or biosimilars are medications that are similar but not identical to the innovator biologics. Despite a paucity of comparative trials, SEBs are poised to enter the Canadian market. They will be prescribed under the same drug name even though they are not identical. This may have implications for patients that are currently on biologic therapy. We conducted a survey of Canadian arthritis patients currently taking innovator biologics to understand their perspectives on SEBs and the possibility of being switched to them.
Methods:
A survey consisting of 14 closed-ended and one open-ended question was administered sequentially to 208 patients at a biologic infusion clinic. In addition to demographic data, patients were asked about their understanding of SEBs, and after providing a definition were asked about factors that might influence them switching to an SEB. The survey results were analyzed using SAS version 9.3.
Results:
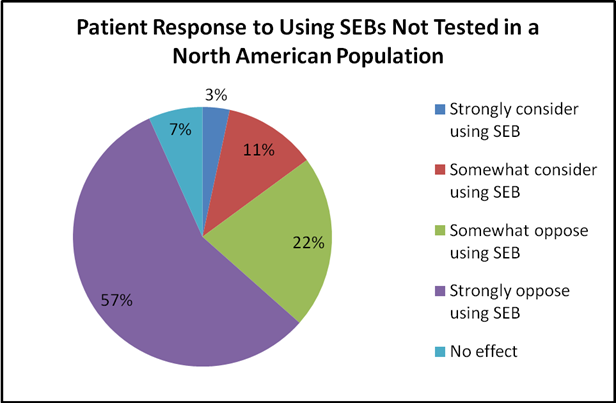
Of the patients surveyed (n=208), mean age was 56 years, 67% were female, 56% of patients had an annual household income of less than $75, 000, and 63% had private drug insurance. The majority of patients had a diagnosis of rheumatoid arthritis (76%) and had been on their current biologic for 1-5 years (55%). Most preferred the subcutaneous route of administration (64%) versus intravenous. When asked about the definition of a SEB, 58% indicated they did not know, 26% chose correctly, and 16% chose incorrectly. When asked about their interest in using a SEB, 30% were neutral, 40% were somewhat or very interested, and 30% were somewhat or completely opposed to it. Potential lower cost of a SEB did not greatly influence this decision, though if an insurance company mandated use due to lower cost most opposed this (54%). The lack of testing in North American patients led to 79% of patients being somewhat or completely opposed to SEBs. Most patients were interested in continuing to use the innovator biologic if there was no further expense (70%) and felt their doctor’s opinion would influence their decision (85%). Demographics, household income, diagnosis and type of current biologic therapy did not affect patient opinions.
Conclusion:
Given the lack of efficacy and safety data for SEBs, there is understandable concern as these medications enter the biologic landscape. This survey identifies a lack of patient understanding of SEBs and highlights a need for further education. Patients are hesitant to use SEBs that are not tested in a North American population and would prefer to stay on innovator biologics if cost was not an issue. Patients value their doctors’ opinions to help them make informed decisions about SEBs. Open dialogue is needed between patients, physicians, industry and regulatory bodies in order to safely introduce SEBs into practice.
Disclosure:
S. Sekhon,
None;
R. Rai,
None;
D. McClory,
None;
C. Whiskin,
None;
M. Deamude,
None;
C. Mech,
None;
L. Vanstone,
None;
A. Shah,
None;
A. N. Lau,
Amgen, Roche,
2,
Amgen, Roche,
8,
Amgen, Roche,
2;
W. Bensen,
Janssen Inc.,
5.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-perspectives-on-the-introduction-of-subsequent-entry-biologics-in-canada/