Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Pragmatic clinical trials (PCTs) allow for study of real world patients using efficient study designs, facilitating comparative effectiveness research in resource constrained settings. Although the need for osteoporosis PCTs is clear, it is unclear what study questions will most impact the field.
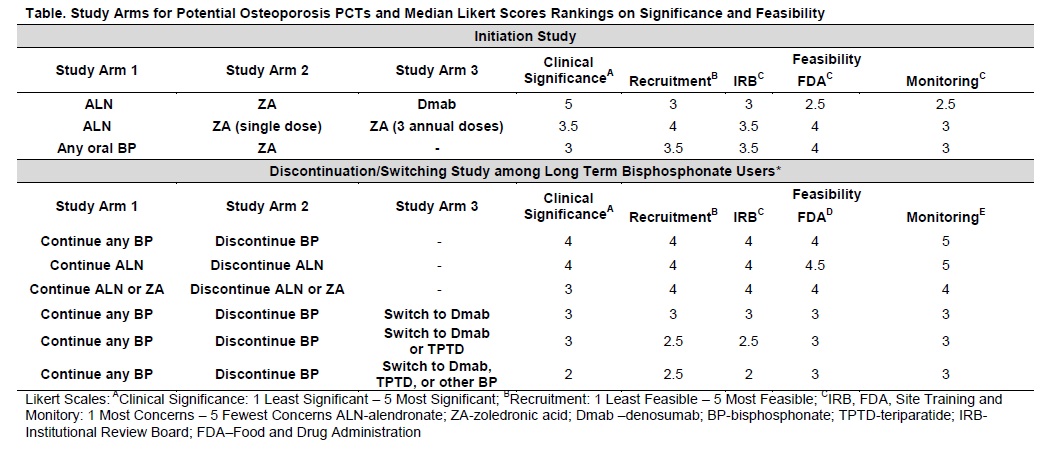
Methods: We conducted a Delphi meeting with 9 experts in osteoporosis trial design and patient care to consider PCT designs with aims of understanding the relative efficacy and safety of osteoporosis drugs. We asked the expert panel to review 2 osteoporosis PCT concepts in a practice-based setting with pre-specified study arms and drugs of interest including 1) active comparator osteoporosis initiation PCT that included 3-arm and 2-arm studies, and 2) osteoporosis therapy discontinuation/switching PCTs that included 2-arm and 3-arm designs among chronic bisphosphonate (BP) users (Table). The primary outcome of both concepts was non-vertebral fracture risk reduction. Participants ranked the clinical significance and feasibility (four domains) of each design (Table). Initial rankings were cast after independent review of the scenarios followed by a webinar discussion and re-ranking of each design.
Results: Rankings associated with initiation studies did not change appreciably pre and post discussion. With 1 being the least and 5 being the most clinically significant, experts ranked the design that compared the fracture reduction of alendronate (ALN), zoledronic acid (ZA), and denosumab (Dmab) as the most clinically significant initiation PCT. The median score was 5 compared to 3.5 for comparing ALN to two strategies of ZA administration (single dose once vs. annual dosing for 3 years), and 3.0 for comparing any oral BP to ZA (Table). Although clinical significance was lower, the panel ranked designs that did not incorporate Dmab as more feasible for recruitment, and human ethics and regulatory approvals (Table). The panel ranked the clinical significance of discontinuation studies with 2-arms higher than designs that incorporated switching (Table). In consideration of switching designs, the panel ranked switching to Dmab more clinically significant than other switching designs. The median recruitment feasibility scores ranged from 2.5-4, lowest designs associated with switching to teriparatide and highest associated with 2-arm studies. The panel ranked human ethics and regulatory concerns greater with switching designs and felt these designs would require more site training and monitoring than 2-arm designs.
Conclusion: The study designs with the highest clinical significance included the 3-arm initiation PCT comparing ALN, ZA, and Dmab and the 2-arm discontinuation PCT that compared continuing any BP or ALN to discontinuing therapy. The 2-arm discontinuation PCTs were also ranked the most feasible, whereas feasibility was higher in other initiation designs.
Disclosure:
N. C. Wright,
Amgen,
2;
A. Oliveira,
None;
A. H. Warriner,
Amylin,
2,
NIH,
2,
AHRQ,
2;
J. R. Curtis,
Roche/Genetech, UCB< Centocor, Corrona,Amgen, Pfizer, BMS, Crescendo, Abbott, 2, Roche/Genetech,UCB, Centocor, CORRONA, Amgen, Pfizer, BMS, Crescendo, Abbott, 5; N. Binkley,
Merck, Amgen, Lilly, Tarsa,
5,
Merck, Amgen, Tarsa,
2;
S. R. Cummings,
None;
M. C. Hochberg,
Abbott Laboratories, Astra-Zeneca, Bioiberica S.A., Eli Lilly Inc., Genentech/Roche, Merck Inc., Novartis Pharma A.G., Pfizer Inc., Stryker LLC, Xoma.,
5;
A. LaCroix,
None;
E. M. Lewiecki,
None;
J. T. Schousboe,
None;
D. H. Solomon,
Amgen,
2,
Abbott Immunology Pharmaceuticals,
2,
Eli Lilly and Company,
2,
Pfizer Inc,
;
R. B. Wallace,
Merck Pharmaceuticals,
5,
Novartis Pharmaceutical Corporation,
5;
K. G. Saag,
Amgen,
2,
Eli Lilly and Company,
2,
Merck Pharmaceuticals,
2,
Novartis Pharmaceutical Corporation,
2,
Amgen,
5,
Eli Lilly and Company,
5,
Merck Pharmaceuticals,
5,
Novartis Pharmaceutical Corporation,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/what-are-the-most-clinically-relevant-and-feasible-pragmatic-osteoporosis-clinical-trial-designs/