Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The cathepsin K inhibitor odanacatib (ODN), a novel antiresorptive that preserves bone formation, is currently in phase 3 development for postmenopausal osteoporosis. In a phase 2 study, 5 years of ODN 50 mg once weekly progressively increased areal BMD at the lumbar spine and total hip (11.9 % and 8.5% from baseline, respectively). ODN reduced bone resorption markers while preserving bone formation markers. In an OVX primate model, ODN has been shown to increase cortical thickness and periosteal bone formation at the central femur and femoral neck.
Methods: In order to determine the effect of ODN on cortical geometry and to estimate bone strength, we conducted a randomized, double-blind placebo-controlled trial, using high resolution quantitative computerized tomography (HR-pQCT) of the distal radius and distal tibia. A total of 214 postmenopausal women, of mean age 64.0 ±6.8 years and baseline lumbar spine T-score -1.81 ±0.83, were randomized to oral ODN 50 mg or PBO weekly for 2 years.
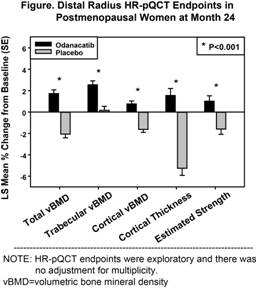
Results: Lumbar spine areal BMD % change from baseline at 1 year (primary endpoint) was statistically significantly greater for ODN than PBO (3.49% treatment difference, p<0.001). After 2 years, there were significantly greater improvements with ODN than PBO in total, trabecular, and cortical volumetric BMD; cortical thickness; and estimated strength (failure load) of the distal radius using HR-pQCT-based finite element analysis (exploratory endpoints, FIGURE). At the radius, odanacatib attenuated the increase in cortical porosity that was seen in the placebo group (treatment difference in least squares mean % change from baseline -7.68, p=0.066). At the distal tibia, changes in volumetric BMD and cortical thickness were similar to changes at the radius. Safety and tolerability were similar between treatment groups.
Conclusion: Odanacatib increased cortical and trabecular density and improved cortical thickness of the distal radius and distal tibia, and improved the estimated bone strength in the distal radius compared to placebo.
Disclosure:
R. Chapurlat,
Merck Pharmaceuticals,
2,
Merck Pharmaceuticals,
5;
K. Brixen,
Merck Pharmaceuticals,
2,
Merck Pharmaceuticals,
5,
Merck Pharmaceuticals,
8;
A. M. Cheung,
Merck Pharmaceuticals,
2,
Merck Pharmaceuticals,
5;
S. Majumdar,
Merck Pharmaceuticals,
2;
B. Dardzinski,
Merck Pharmaceuticals,
1,
Merck Pharmaceuticals,
3;
A. Cabal,
Merck Pharmaceuticals,
1,
Merck Pharmaceuticals,
3;
N. Verbruggen,
Merck Pharmaceuticals,
1,
Merck Pharmaceuticals,
3;
S. Ather,
Merck Pharmaceuticals,
1,
Merck Human Health,
3;
E. Rosenberg,
Merck Pharmaceuticals,
1,
Merck Pharmaceuticals,
3;
A. de Papp,
Merck Pharmaceuticals,
1,
Merck Pharmaceuticals,
3.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-odanacatib-on-the-distal-radius-and-tibia-in-postmenopausal-women-improvements-in-cortical-geometry-and-estimated-bone-strength/