Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Fibromyalgia (FM) affects 2-5% of adults in the United States. Pregabalin (antiepileptic drug [AED]), and duloxetine and milnacipran (serotonin and norepinephrine reuptake inhibitors), are approved to treat FM. FM treatment guidelines also include gabapentin (AED), amitriptyline (tricyclic antidepressant), selective serotonin reuptake inhibitors (SSRIs), and tramadol; in general, opioids, particularly strong opioids, are not recommended. This study evaluated treatment patterns of recommended and non-recommended medications among patients newly diagnosed with FM.
Methods: This retrospective study used medical and pharmacy claims data and enrollment information for adult commercial health plan members of a large US health plan. Patients had ≥2 medical claims with a diagnosis (dx) of FM from January 2008-February 2009; the date of the first FM dx was the index date. Patients also had: 6 months of pre-index and 12 months of post-index continuous enrollment; no pre-index FM dx; and ≥1 pharmacy claim for an FM guideline medication (pregabalin, gabapentin, duloxetine, milnacipran, amitriptyline, or SSRI) or for an opioid on or within 6 months after the index date. The date of the first medication (Rx) was the treatment date. The principal outcomes were indicators identifying patients with ≥1 fill of an FM guideline Rx or opioid during each 3-month interval (quarter) of the 12-month post-index period. Descriptive analysis was conducted.
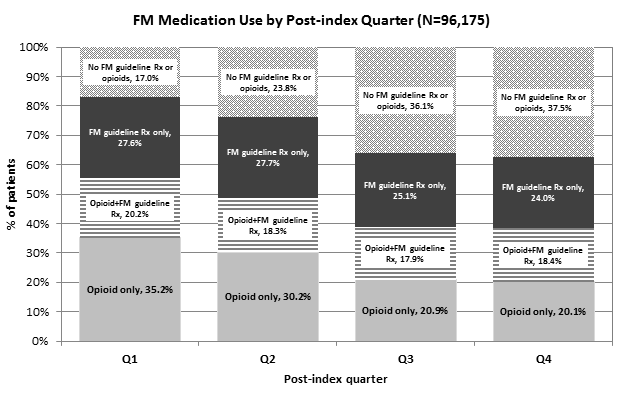
Results: The study sample was 96,175 patients with mean age 47.3 years and 72.5% female. Fifty-six percent of patients were prescribed opioids on their treatment dates and 44% received an FM guideline Rx; 17% of opioid recipients were prescribed tramadol. The figure shows that 55% of patients were prescribed opioids only or opioids as well as FM guideline Rx in the first quarter after FM dx. The percentage of patients with FM guideline Rx (with or without opioids) was consistent through the post-index period. The percentage of patients who received opioids only decreased over time. However, >20% of patients were treated only with opioids in each quarter and an additional 18% received opioids in addition to FM guideline Rx.
Conclusion: In the 12-month post-index, a substantial proportion of patients were prescribed opioids and more than half did not receive FM guideline Rx. These real-world utilization results indicate that an opportunity may exist for improved FM management using recommended therapies in clinical practice.
Disclosure:
S. N. Shah,
Pfizer, Inc.,
1,
Pfizer, Inc.,
3;
R. Halpern,
Pfizer, Inc.,
9;
J. C. Cappelleri,
Pfizer Inc.,
1,
Pfizer, Inc.,
3;
E. T. Masters,
Pfizer, Inc.,
1,
Pfizer Inc,
3;
A. G. Clair,
Pfizer, Inc.,
1,
Pfizer Inc,
3;
C. Blauer-Peterson,
Pfizer Inc,
9;
D. Van Voorhis,
Pfizer Inc,
9.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/examination-of-patients-newly-diagnosed-with-fibromyalgia-use-of-guideline-recommended-therapies-and-opioids-in-clinical-practice/