Background/Purpose: Biologic DMARDs (bDMARDs) are effective yet expensive treatments for RA. Variability in the prescribing practices of rheumatologists using these agents may be attributable to patient-specific clinical factors. We performed a quality improvement project to better understand this variability, comparing practice to an adapted standardized treatment pathway.
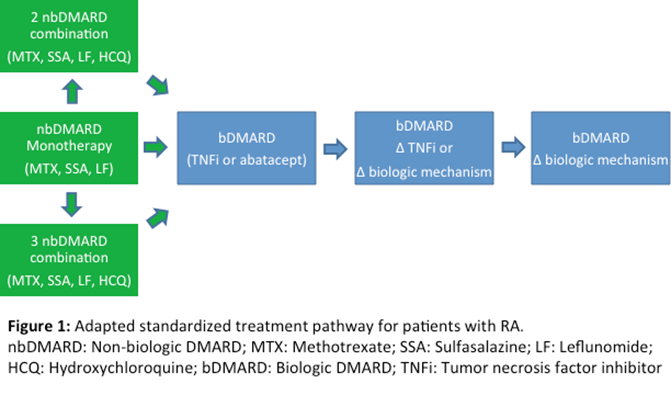
Methods: A standardized treatment pathway was developed based on the 2012 ACR guidelines for treatment of patients with early moderate to severe RA (Singh JA et al., 2012) and was endorsed by the rheumatologists in our practice (Figure 1). This pathway differed from the ACR guidelines primarily by liberalizing the duration required for each treatment step.
We reviewed charts of 224 patients with ³ 3 ICD 9 codes for RA (714.0) seen at our single center academic practice who initiated a new bDMARD (first time use or a change from prior bDMARD) from January through December 2013. Data were abstracted using a standardized form, including demographics, RA characteristics, complete available medication history and reasons for medication choices. Each patient was included only once. We categorized patients as having treatment courses that did or did not follow the treatment pathway. Inter-rater reliability (kappa), assessed by re-review of charts of patients with treatment courses that varied from the pathway, was 0.82, p < 0.001. When reviewers disagreed, final adjudication occurred through a consensus process.
Results:
224 patients initiated a new bDMARD during the study period. Mean age was 53 (range 20-84) and 183 (82%) were female. 197 (88%) had disease duration of over 2 years. Erosive disease was seen in 99 (44%) and 116 (52%) were seropositive. Only 13 patients (6%) had treatment courses that did not follow the treatment pathway (Table 1). The reasons for these variations included co-morbidities (n = 8), disease severity (n = 1) and initial care at other institutions (n = 4). None of the included patients had failed oral triple therapy prior to initiation of a biologic.
Conclusion:
We examined practice patterns for the prescription of bDMARDs at our institution and found little variability based on an adapted standardized treatment pathway. Variations from the pathway were justified by patient co-morbidities and disease characteristics, suggesting that existing variability may be appropriate.
|
Table 1: Reasons for patient treatment not following suggested pathway (N=13 out of 224) |
||
|
Deviation |
Number |
Reason |
|
Etanercept as initial DMARD |
8 |
Therapy started at another institution (4) Co-morbidity considerations (4) -Liver disease (1) -Lung/liver disease, sulfa allergy (1) -Pregnancy/post-partum breastfeeding (2) |
|
Infliximab + methotrexate as initial DMARDs |
1 |
Severe symptoms with high disease activity (1) |
|
Rituximab as initial biologic DMARD |
4 |
Co-morbidity considerations (4) -Multiple sclerosis (1) -Active leiomyosarcoma (1) -Interstitial lung disease (1) -Latent tuberculosis (1) |
Disclosure:
H. O. Tory,
None;
J. A. Awosogba,
None;
A. J. Dave,
None;
J. S. Coblyn,
CVS caremark,
5;
D. H. Solomon,
None;
S. P. Desai,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/variation-in-the-prescribing-practices-of-biologic-dmards/