Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: IA steroids are used to treat knee OA. Little is known about the systemic effect of intraarticular steroid injections on bone or the hypothalamic-pituitary- adrenal axis (HPA).
Methods: We describe a 2:2:1 randomized, double blind, placebo controlled study. 25 subjects (20 females, 5 males) with knee OA, age range 45–83 years were identified from our clinical practice. Ten subjects (Group 1) were injected with Depo-methylprednisolone 80 mg plus lidocaine 20 mg, 10 additional subjects (Group 2) were injected with Depo-methylprednisolone 16 mg plus lidocaine 20 mg and 5 subjects (Group 3) were given normal saline with lidocaine 20 mg. Blood draws were performed 5 times (Days 0, 2–3, 7, 14 and 28). Means and 95% CI were reported by time and group. All plots show mean (+/- SEM). Between and within-group comparison p-values were generated by t-tests. All parametric assumptions were verified.
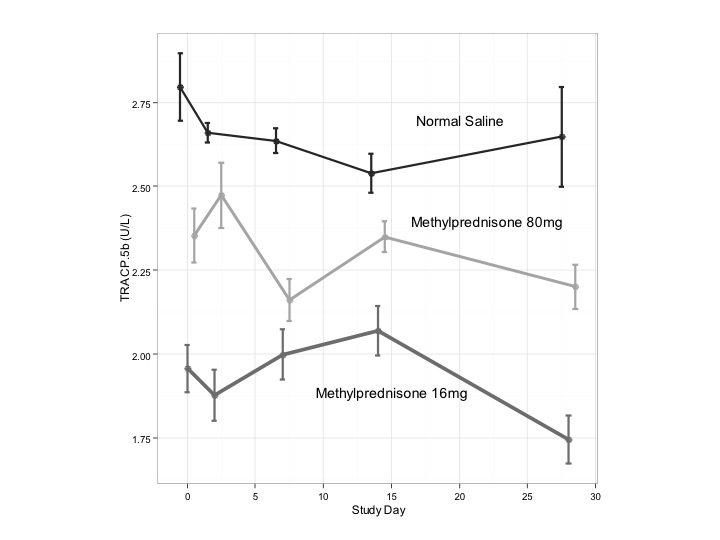
Results: Mean levels of serum osteocalcin, a bone formation marker, reached a nadir (10.2 ng/ml (95% CI 9.3-11.2) from baseline of 15.5 ng/ml (95% CI 14.4-16.7) by Days 2–3 with recovery by Day 7 (14.9 ng/ml (95% CI 13.8-16.1)) after IA corticosteroids only for Group 1, with mean change of 5.3 ng/ml (95% CI 2.4-8.2) (p = 0.01). No change was seen in Groups 2, 3 (Fig 1, p> 0.05). In contrast, the bone catabolic marker Tartrate-resistant Acid Phosphatase Form 5b (TRACP-5b) showed no consistent change for any of the three groups over 28 days (range 1.7–2.7 U/L) (Fig 2). Mean baseline endogenous cortisol level reached a nadir by Days 2-3 for Group 1 (8.9 mcg/mL (95% CI 8.0-9.8)) and by Day 7 for Group 2 (8.9 mcg/ml (95% CI 8.0-9.7). Both returned at least to baseline by day 14. Baseline vitamin D levels did not differ significantly between groups (p > 0.05), though Group 1 levels were not as low (range 22–47 ng/mL) as Group 2 (range 8.3–51.5 ng/mL) or Group 3 (range 8.6–38.5 ng/mL). In control subjects, lower baseline levels of vitamin D were associated with greater decreases in osteocalcin at Day 2 – 3 (r = 0.9, p < 0.05). Low levels of vitamin D were associated with greater levels of suppression of osteocalcin at Day 7 in Group 2 (r = -0.7, p = 0.03).
Conclusion: IA corticosteroids have a transient adverse effect on bone formation with significant recovery of osteocalcin levels by one week and no change in bone catabolism. Cortisol levels decrease slightly at one week of IA administration and rebound above baseline by two weeks. In contrast to daily oral corticosteroids, single doses of IA steroids have no persistent adverse effect on bone or the HPA axis.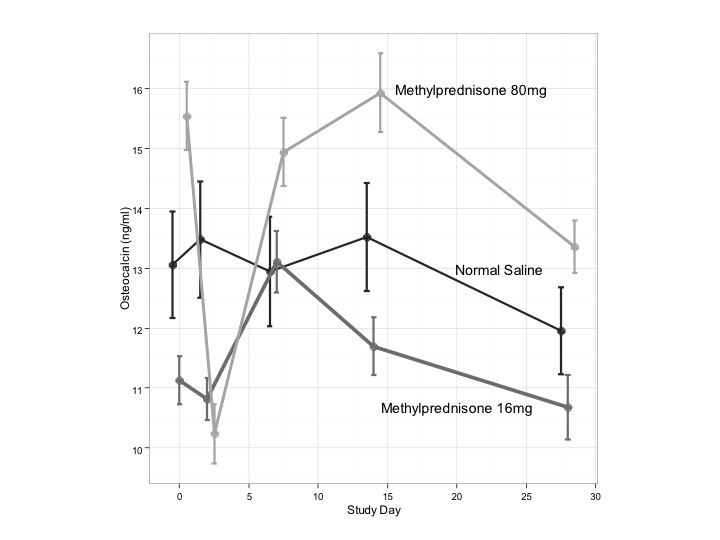
Disclosure:
M. Imran,
None;
A. Baratham,
None;
J. Wick,
None;
B. Lukert,
None;
H. Lindsley,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-intraarticular-ia-corticosteroid-injections-on-bone-markers-and-endogenous-cortisol-in-patients-with-knee-osteoarthritis-oa-a-randomized-double-blind-placebo-controlled-trial/
