Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Treatment options for systemic JIA (sJIA) have recently expanded to include IL1 and IL6 inhibitors in addition to traditional treatments such as glucocorticoids (GC) and methotrexate (MTX). The Childhood Arthritis and Rheumatology Research Alliance (CARRA) developed standardized consensus treatment plans (CTPs) for new onset sJIA to study comparative effectiveness of these treatments using an observational registry. A pilot study was conducted to assess the feasibility of using the CTPs for this purpose.
Methods:
New onset untreated sJIA patients (pts) enrolled in the CARRA Registry are treated according to the CTP selected by the treating physician (GC alone; MTX ± GC; IL1 inhibitor (IL1i) ± GC; IL6 inhibitor (IL6i) ± GC). Data is collected at standard intervals for 9 mos from baseline. Physicians could deviate from the chosen CTP for inadequate response at any time. If GC is started, the CTPs suggest an aggressive taper (<50% of the starting dose by 3 mos). Biospecimens are being collected from consenting pts for future use.
Results:
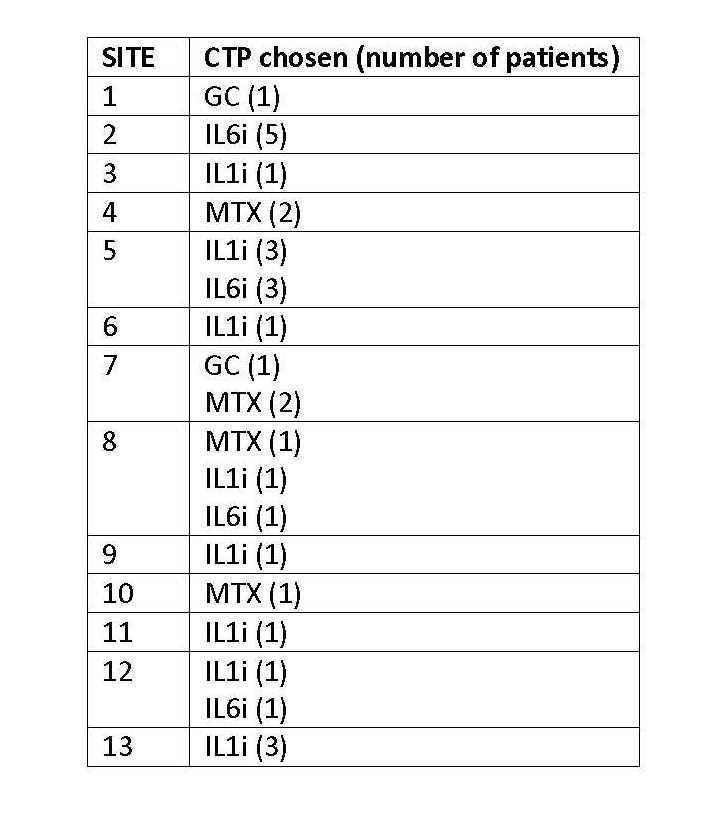
13 of 15 CARRA study sites enrolled at least 1 pt (total 30, range 1-6 pts/site) (Table 1). As of 6/2014, 8 (27%) of 30 pts had reported completing the study. The GC-only CTP was used in only 2 pts (7%) and a biologic was used as initial therapy in 22 (73%). The chosen CTP varied by site, especially with regard to use of biologics vs non-biologics (Table 2). Most common reasons cited for choosing non-biologic CTPs were: usual treatment at site (63%), perceived safer treatment (63%), and pt preference (38%); most common reasons for choosing biologic CTPs: will likely work best (77%), better tolerated (59%), and perceived safer (45%). As of 6/2014, 13 pts (43%) had changed or deviated from the starting CTP. 67% of patients did not consent for specimen collection.
Conclusion:
The CARRA sJIA CTP pilot study successfully reached its target enrollment. The study is ongoing for most patients. Despite the absence of randomization, CTP choice is reasonably balanced aside from the GC CTP and appears to be based on physician/center preference. Most providers found the CTPs acceptable to use, and enrollment of additional patients at more CARRA sites to study comparative effectiveness of the CTPs appears feasible. Exploring barriers and improving processes for biospecimen collection consent may increase participation in future studies.
Table 1: sJIA Patients Enrolled in CTP Pilot Study
Table 2: Selection of CTP by Site
Disclosure:
Y. Kimura,
Novartis Pharmaceutical Corporation,
2,
Novartis Pharmaceutical Corporation,
5;
E. Morgan-DeWitt,
None;
K. L. Mieszkalski,
None;
T. B. Graham,
None;
T. Beukelman,
Novartis Pharmaceutical Corporation,
5,
Genentech and Biogen IDEC Inc.,
5,
UCB,
5,
Pfizer Inc,
2;
M. F. Ibarra,
None;
N. T. Ilowite,
Genentech and Biogen IDEC Inc.,
5,
Genentech and Biogen IDEC Inc.,
8,
Janssen Pharmaceutica Product, L.P.,
5,
Janssen Pharmaceutica Product, L.P.,
9,
Novartis Pharmaceutical Corporation,
5,
Novartis Pharmaceutical Corporation,
9;
M. S. Klein-Gitelman,
None;
K. Onel,
None;
S. Prahalad,
None;
M. G. Punaro,
None;
S. Ringold,
None;
D. Toib,
None;
H. Van Mater,
None;
P. F. Weiss,
None;
L. Schanberg,
Novartis Pharmaceutical Corporation,
2,
UCB Pharma,
5,
Eli Lilly and Company,
5.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/preliminary-results-from-the-childhood-arthritis-and-rheumatology-research-alliance-systemic-jia-consensus-treatment-plans-pilot-study/