Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Systemic JIA (sJIA) is a rare disease whose treatment has changed in the past 10 yrs. The Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry contains a large cohort of sJIA pts. We aimed to: (1) describe the characteristics of the CARRA Registry sJIA cohort; (2) identify medication usage trends; and (3) identify subgroups at increased risk for poor outcomes.
Methods:
54 US/Canadian sites enrolled 528 sJIA pts as a cross-sectional convenience sample from 2010-2013. Only pts with complete datasets were included in this analysis. We tested binary and continuous variables for subgroup differences among or across groups, using a chi-square or ANOVA, respectively.
Results:
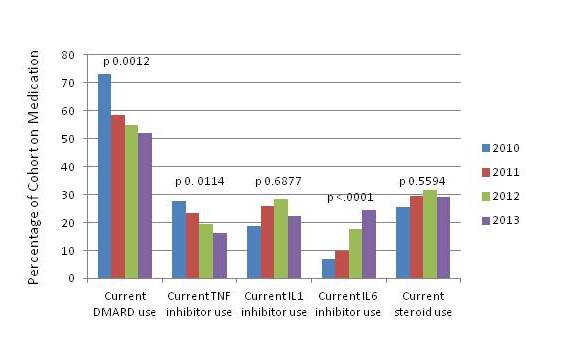
435 pts were included (Table 1). Disease activity was low: 15% had rash, 7% fever, median joint count 0, and median physician global assessment 1. Significant changes in medication usage occurred over the study period: DMARD and TNF inhibitor use decreased while IL-6 inhibitor (IL-6i) use increased (Fig 1). 29% were on corticosteroids at enrollment. African Americans (AA) had higher CHAQ, worse quality of life and poorer ACR functional class (p 0.0004). Pts diagnosed at a younger age (< 2 yrs) had more frequent biologic use and lower overall well being. Joint damage on imaging increased with younger age at diagnosis (p 0.0003). 259 pts had follow-up visits at least 3 mos from enrollment, and disease activity measures improved in these pts. Of 234 pts without active systemic features, 91 had active arthritis (median active joints=4). There were no other disease or demographic differences in the subset with persistent arthritis, but there was increased current IL-6i, steroid and NSAID use and past biologic use compared to those without persistent arthritis.
Conclusion:
This study describes the largest sJIA cohort reported to date. Significant changes occurred in medication usage over the study period, but corticosteroids are still frequently used. AA pts had more severe disease, as did pts diagnosed at a younger age. A significant proportion has persistent arthritis despite new treatments. Predictors of persistent arthritis are needed to improve treatment and outcomes in this subgroup.
Table 1. Demographic Features (n=435)

Figure 1: Current Medication Usage by Year of Visit (baseline and follow-up visits)
Disclosure:
G. L. Janow,
None;
L. Schanberg,
Novartis Pharmaceutical Corporation,
2,
UCB Pharma,
5,
Eli Lilly and Company,
5;
S. Setoguchi,
Novartis Pharmaceutical Corporation,
2;
E. D. Mellins,
Novartis Pharmaceutical Corporation,
2,
Ascendant ,
5,
Five Prime,
5;
R. Schneider,
Novartis Pharmaceutical Corporation,
2,
Novartis Pharmaceutical Corporation,
5,
Roche Pharmaceuticals,
5;
Y. Kimura,
Novartis Pharmaceutical Corporation,
2,
Novartis Pharmaceutical Corporation,
5;
T. CARRA Registry Investigators,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/demographic-clinical-and-treatment-characteristics-of-the-childhood-arthritis-and-rheumatology-research-alliance-registry-systemic-jia-cohort/
