Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: A previous analysis of RA patients in national administrative databases of the Department of Veterans Affairs (VA) suggested that injectable MTX was associated with a higher likelihood to remain on MTX monotherapy. We seek to determine whether RA patients in the VA database treated with injectable MTX after being unable to tolerate or having inadequate response to oral MTX remain on MTX monotherapy longer than those treated only with oral MTX. We also compare the rates of liver enzyme abnormalities between patients using injectable MTX monotherapy and those using oral MTX monotherapy.
Methods: The duration of MTX monotherapy before a therapeutic change was required (defined as switching to or adding another DMARD or biologic agent) was compared between patients treated with injectable MTX for at least 30 days and those who were treated only with oral MTX using a Wilcoxon-Matt-Whitney test. Factors that could potentially influence the duration of MTX monotherapy in subjects with therapeutic change included the use of injectable MTX, gender, age, race, starting MTX dose, maximum MTX dose, and modified Charlson comorbidity score. These factors were assessed using a log-rank test and Kaplan-Meier curves; in order to correct for patient variables, linear regression models were also run. Liver enzyme abnormalities were compared between patients on injectable and oral MTX who received MTX monotherapy for >90 days; abnormal alanine aminotransferase (ALT) levels and aspartate aminotransferase (AST) levels were defined as exceeding twice the upper limit of normal.
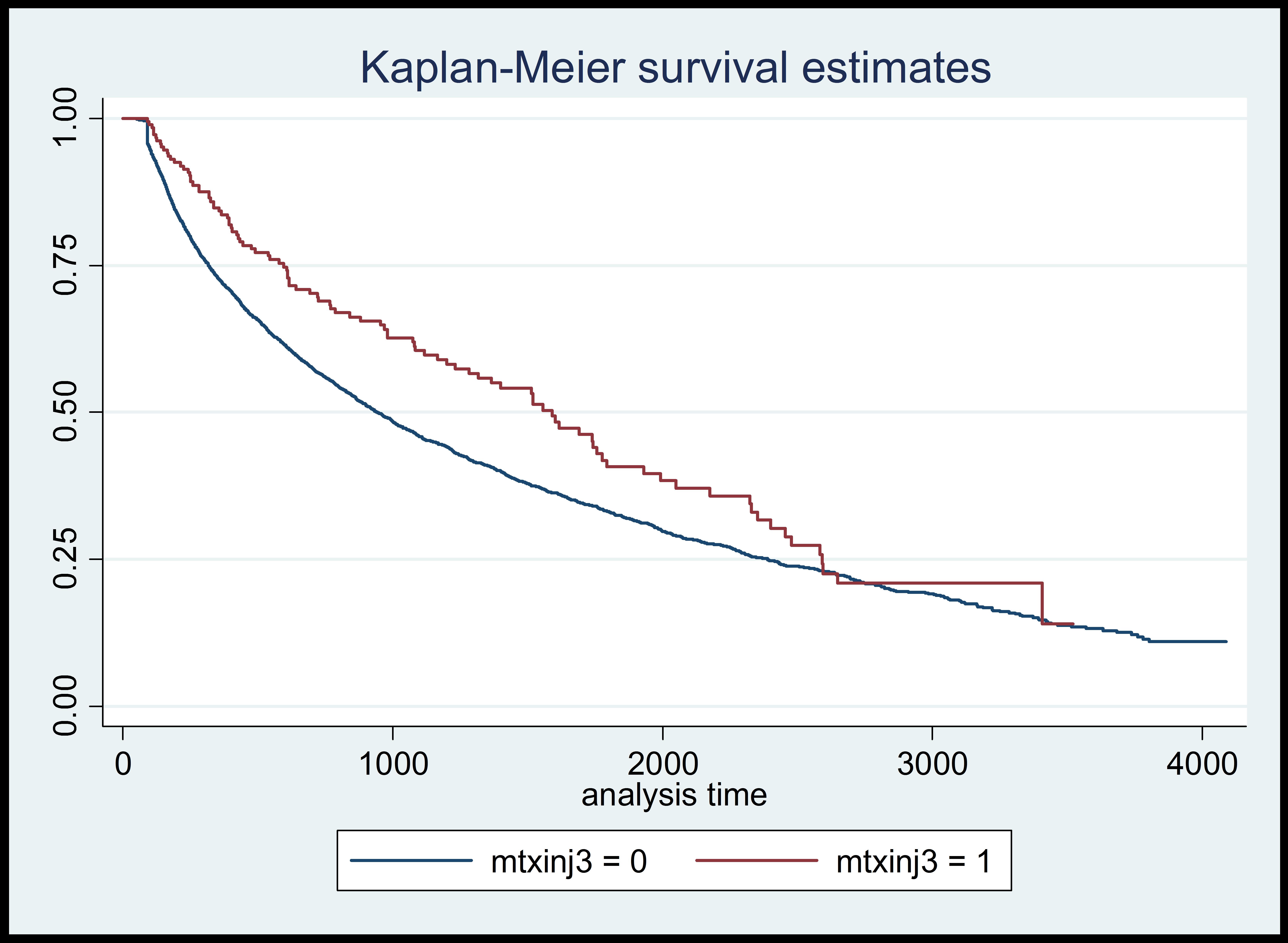
Results: Of the 7107 patients who were treated with MTX monotherapy for >90 days, 3910 required a therapeutic change (3808 were treated with oral MTX, 102 with injectable MTX). Patients treated with oral MTX remained on MTX monotherapy for a mean (SD) of 627 (636.5) days compared with 962 (786.6) days for patients treated with injectable MTX monotherapy (P<0.001). Based on log-rank tests (see figure) and linear regression models, the use of injectable MTX was significantly associated with longer duration of MTX monotherapy (P=0.0018 and P<0.001, respectively). When adjusted for patient variables, the duration of MTX monotherapy was also significantly associated with age, race, starting MTX dose, and maximum MTX dose. Of the patients treated with MTX who also had ALT and/or AST levels assessed, the rates of abnormal ALT and AST levels were not significantly different between those receiving oral and injectable MTX (P=0.870 and P=0.056, respectively).
Conclusion: Among patients identified in the VA database, the use of injectable MTX was associated with a significantly longer duration of MTX monotherapy compared with oral MTX. No significant differences in liver enzyme abnormalities were found between patients treated with injectable MTX and oral MTX.
Disclosure:
B. Ng,
Antares Pharma,
2;
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/factors-associated-with-methotrexate-treatment-duration-including-subcutaneous-use-in-patients-with-rheumatoid-arthritis-observations-from-the-va-database/