Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: There is a growing interest about optimization of biological therapies but for now no strong evidence is available to support a tapering strategy in clinical practice.
Our aim was to compare the clinical outcomes of a tapering strategy to the standard dosing regimen of TNF inhibitors (TNFi) in patients with rheumatoid arthritis (RA) and low disease activity during long-term follow-up.
Methods: In this retrospective observational study, two groups of RA patients on TNFi with DAS28<3.2 were compared: the tapering group (TG: 67 pts from Spain) and the control group with the standard therapy regimen (CG: 77 pts from the Netherlands). DAS28 was measured at different time points: visit 0 (prior starting TNFi), visit 1 (prior to starting tapering in TG and at least 6 months with DAS28<3.2 after starting TNFi in the TG and CG), visit 2 (6 months after visit 1), visit 3 (1 year after visit 1) and visit 4 (the last visit available after visit 1).
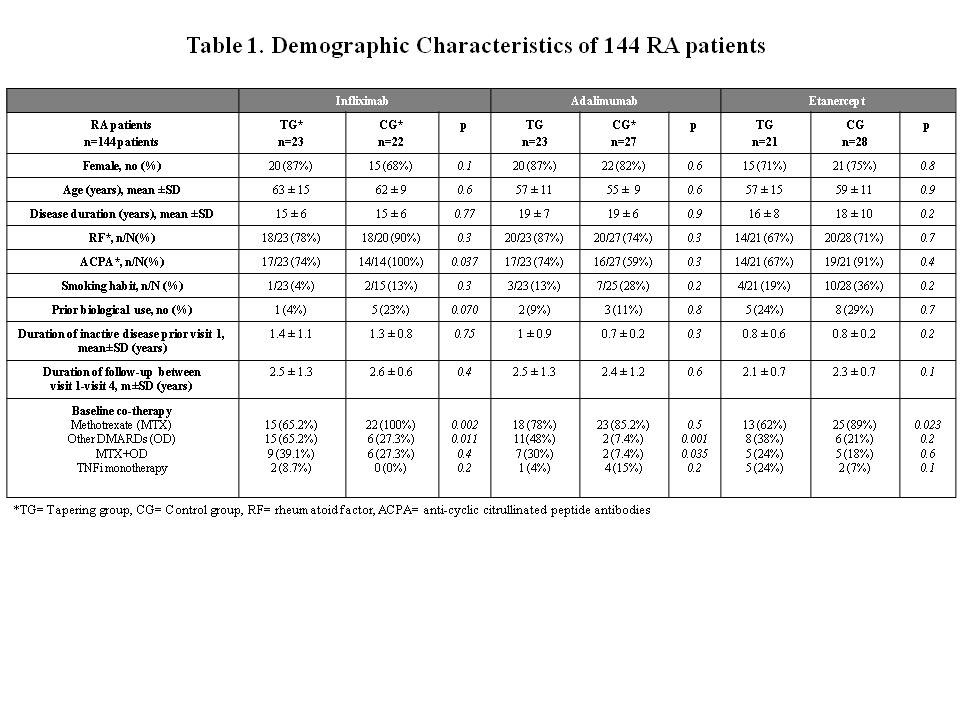
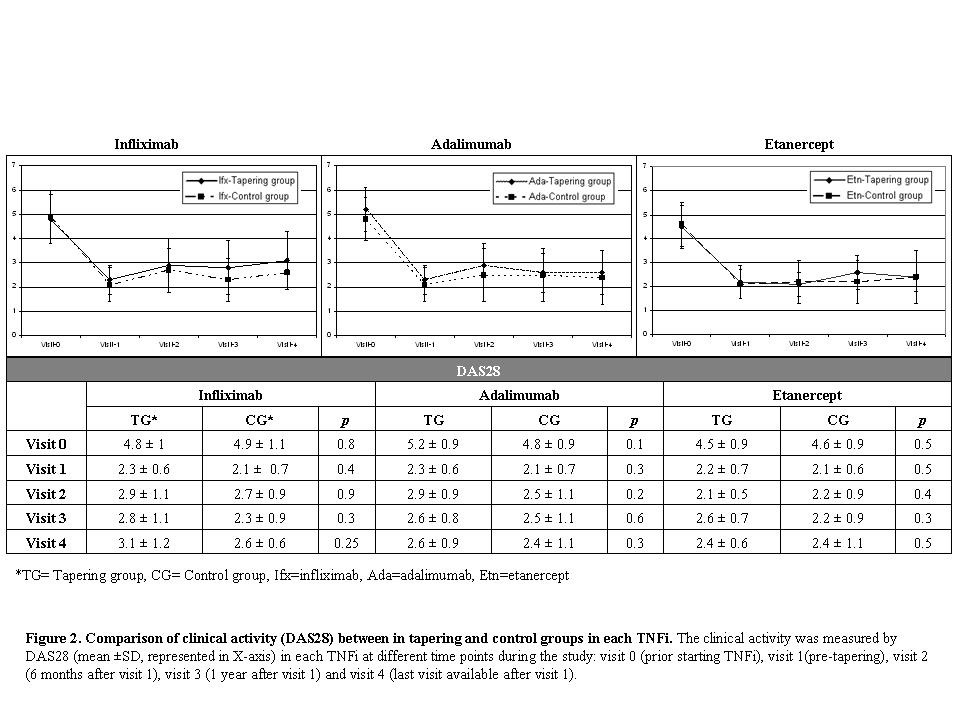
Results: The demographic characteristics are summarized in Table 1. Despite an overall reduction of administered drug at visit 4 in the TG (an interval elongation of 32.8% in infliximab, 52.9% in adalimumab and 52.6% in etanercept), no significant differences were found in the clinical activity between the groups at the end of the study (DAS28: 2.7±0.9 in TG vs. 2.5±1 in CG, p=0.1) (Figure 2). The number of patients with flares was similar in both groups [28/67 (42%) in TG vs. 36/75 (48%) in CG, p=0.5]. No significant differences were seen in the proportions of patients who dropped out [10/67 (15%) in TG vs. 6/77 (8%) in CG, p=0.17].
Conclusion: The tapering strategy of TNFi in RA patients with low disease activity results in an important reduction in the amount of drug administered, while the disease control remains similar to that of patients on the standard dosing regimen.
Disclosure:
C. Plasencia-Rodriguez,
Pfizer Inc,
2;
G. J. Wolbink,
Pfizer, Amgen,
8;
C. L. M. Krieckaert,
None;
E. L. Kneepkens,
None;
S. Turk,
None;
M. G. Bonilla,
None;
A. Villalba,
None;
D. Peiteado,
None;
L. Nuño,
None;
M. T. Nurmohamed,
None;
T. Rispens,
None;
D. van der Kleij,
None;
T. Jurado,
None;
E. Martín-Mola,
None;
D. Pascual-Salcedo,
Pfizer Inc,
2;
A. Balsa,
Pfizer Inc,
9.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparing-a-tapering-strategy-to-the-standard-dosing-regimen-of-tnf-inhibitors-in-patients-with-rheumatoid-arthritis-in-remission-or-with-low-disease-activity/