Background/Purpose
The OMERACT PMR specialist interest group was established to develop a core outcome measurement set for PMR using the methods of OMERACT filter 2.0. This work builds on previous work undertaken to identify potential domains, which were presented at OMERACT 11.
Methods
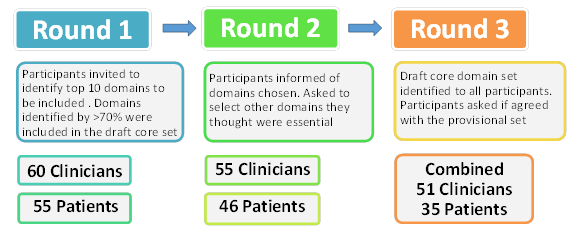
A three-round Delphi survey was undertaken to identify domains of importance (Figure1). Additionally, meetings of patient and clinician participants were convened in order to scrutinise and finalise the candidate core domain set. A review of the PMR literature was undertaken to identify outcome measures and instruments used in previous PMR research. The candidate domains and identified instruments were presented and discussed at OMERACT 12.
Figure 1.
Results
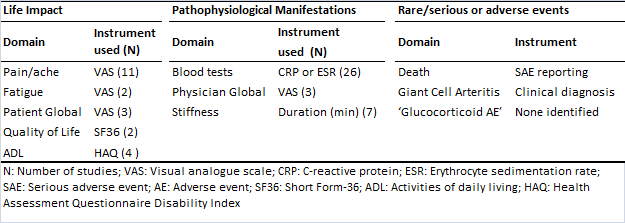
The literature review identified 28 studies for full review. The identified domains from the Delphi survey and corresponding measurement instruments are presented in Table 1.
Table 1.
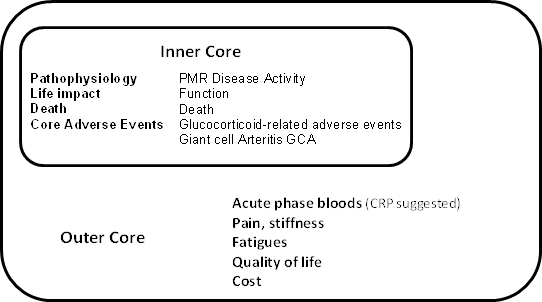
No study reported any patient involvement in the development of the outcome measures used. The candidate core domain set emerging after discussion at the OMERACT 12 PMR special interest group is shown in figure 2. Two studies undertook instrument validation and demonstrated poor test-retest reliability for fatigue VAS, morning stiffness duration, and SF36 mental component score and that the HAQ performed well for PMR.
Figure 2.
 |
Conclusion
The concern over glucocorticoid (GC) adverse effects (AEs) warrants their inclusion in the core set. No accepted measurement instrument for GC AEs was identified. GC AEs were not routinely reported in the studies identified. Review work by a EULAR Task Force found little evidence of their prevalence and severity at different daily and cumulative doses. Low dose GCs remain the cornerstone of PMR treatment and so the reporting of relevant associated AEs should be routine. The candidate core domain set is supported by substantial patient and clinician contributions, and by the previous use of these domains. Full evaluation of the instruments would now be worthwhile.
Disclosure:
S. Mackie,
None;
T. Helliwell,
None;
R. Hughes,
None;
E. Brouwer,
None;
C. T. Pease,
None;
C. Mallen,
None;
M. Boers,
None;
J. R. Kirwan,
Horizon Pharma USA Inc,
2.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/core-outcome-domains-and-potential-measurement-instruments-in-polymyalgia-rheumatica-pmr-using-omeract-filter-2-0/