Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is implicated in RA pathogenesis via myeloid and granulocyte cell lineage activation. In a prior Phase 2a study (NCT01050998), mavrilimumab, a first-in-class inhibitor of the GM-CSF receptor α showed a rapid and sustained effect over the 12-week study duration. Here, we present results of a 24-week Phase 2b randomized, double-blind, placebo (PBO)-controlled, parallel-group, multicenter study (NCT01706926) that evaluated the efficacy and safety of mavrilimumab in patients with moderate-to-severe adult-onset RA.
Methods
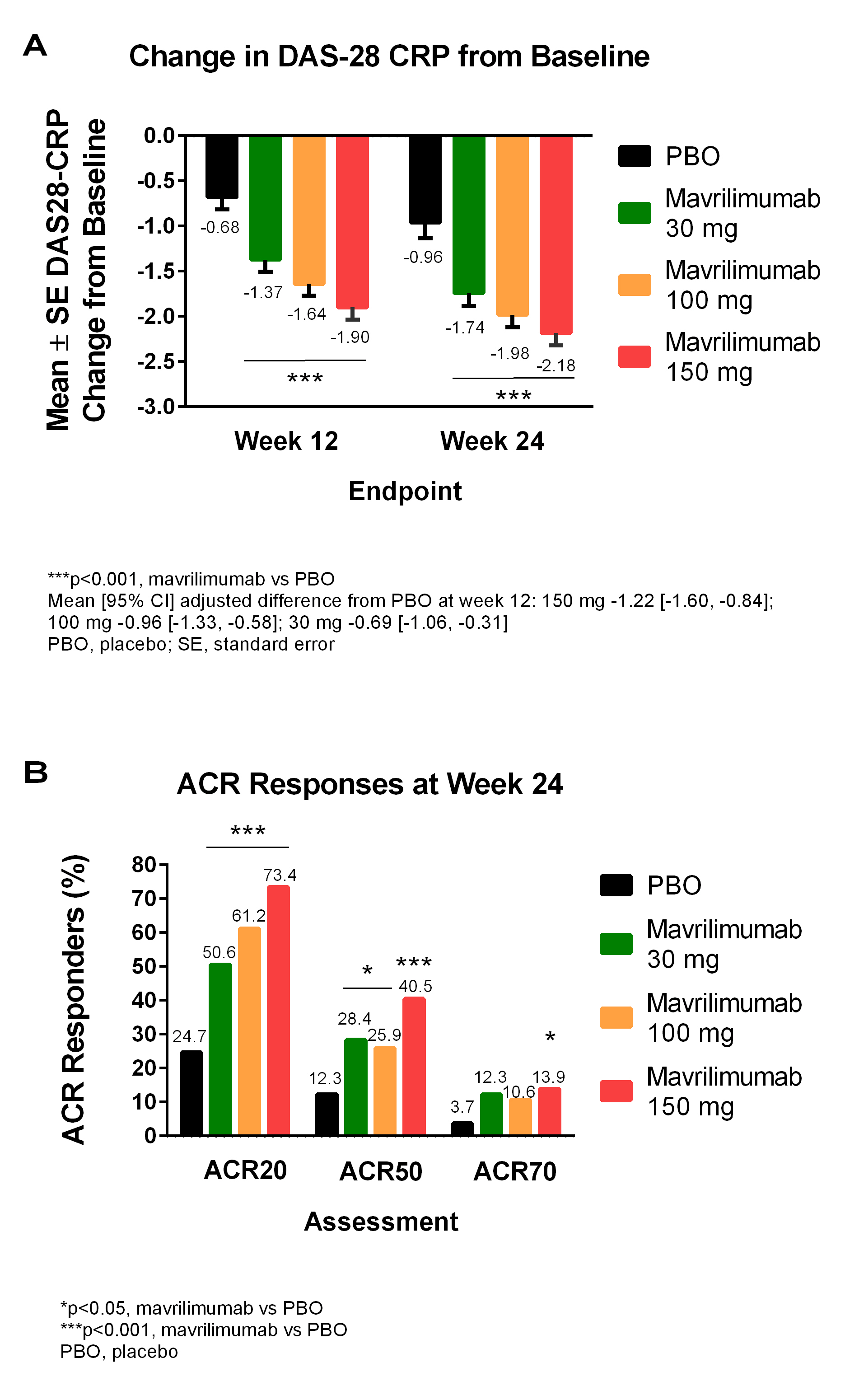
Patients (18–80 years; inadequate response to ≥1 DMARD; DAS28-CRP ≥3.2; ≥4 swollen joints; receiving methotrexate [MTX]) were randomized 1:1:1:1 to receive 1 of 3 subcutaneous mavrilimumab doses (30, 100, 150 mg) or PBO every 2 weeks plus MTX (7.5–25.0 mg/week) for 24 weeks. Co-primary endpoints were change in DAS28-CRP (day 1 to week 12) and ACR20 response rate (week 24). Safety and tolerability were measured through assessment of adverse events (AEs) and pulmonary parameters. Results were analyzed using the modified intent-to-treat population.
Results
In total, 326 patients from Europe, South America and South Africa (mean [SD] age 51.8 [11.1] years; female 86.5%; mean [SD] DAS28-CRP 5.8 [0.9]; RF/ACPA+ 81.9%) were randomized to PBO or mavrilimumab 30, 100, or 150 mg (n=81, 81, 85, 79, respectively). At week 12, a statistically significant difference in DAS28-CRP (p<0.001) was seen for all doses of mavrilimumab vs PBO (Figure A). At week 24, a significantly higher percentage of all mavrilimumab-treated patients also met the ACR20 co-primary endpoint vs PBO (Figure B). A significant (p<0.001) separation vs PBO for these parameters was seen as early as week 1 for mavrilimumab 150 mg, which was also associated with significantly higher ACR50 and ACR70 response rates vs PBO at week 24 (Figure B). A dose response effect was observed across multiple secondary endpoints, with separation vs PBO evident from week 1 and 1 dose. The most common treatment-emergent AEs were headache (2.5%, 6.2%, 4.7%, 7.6%), nasopharyngitis (7.4%, 4.9%, 3.5%, 7.6%) and bronchitis (7.4%, 3.7%, 1.2%, 5.1%) for PBO and 30, 100, or 150 mg, respectively. There was no increase in pulmonary AEs for mavrilimumab vs PBO (6.2%, 3.5%, 6.3% vs 9.9%). No serious infections were observed in the 100 and 150 mg groups; 2 cases of pneumonia were seen (30 mg and PBO groups). There were no deaths or anaphylaxis, and no apparent dose relationship for AEs. >90% patients entered a long-term open-label study.
Conclusion
This second Phase 2 study demonstrates the potential benefit of inhibiting macrophage activity via the GM-CSFRα pathway on RA disease activity. The study met both co-primary endpoints with a clear dose response effect. An acceptable tolerability profile was demonstrated over the 24-week study period.
Disclosure:
G. Burmester,
Medimmune,
5;
I. B. McInnes,
Medimmune,
5,
Medimmune, AstraZeneca,
5;
J. M. Kremer,
Corrona,
1,
Abbvie, Amgen, Genentech, Lilley, Pfizer,
2,
Abbvie, Amgen, BMS, Genentech, Lilley, Pfizer,
5;
P. Miranda,
Medimmune,
2;
M. Korkosz,
None;
J. Vencovsky,
None;
A. Rubbert-Roth,
None;
E. Mysler,
Medimmune,
2;
S. Sandbach,
None;
M. A. Sleeman,
AstraZeneca,
1,
Medimmune,
3;
A. Godwood,
AstraZeneca,
1,
Medimmune,
3;
D. Close,
Medimmune,
3;
M. Weinblatt,
BMS, UCB, Crescendo Bioscience,
2,
Medimmune, AstraZeneca, Amgen, Abbvie, BMS, Crescendo Bioscience, Lilly, Pfizer, UCB, Roche,
5.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safetytolerability-of-mavrilimumab-a-human-gm-csfra-monoclonal-antibody-in-patients-with-rheumatoid-arthritis/