Session Information
Session Type: Abstract Submissions (ACR)
 Background/Purpose: Tocilizumab (TCZ), anti-human interleukin-6 receptor monoclonal antibody, was the first biologic agent used in the treatment of systemic juvenile idiopathic arthritis (sJIA) in Japan. We have previously reported that long-term TCZ therapy could induce drug-free remission in sJIA patients resistant to conventional prednisolone (PSL) therapy1). On the other hand, extremely high levels of serum IL-18 (>10,000 pg/ml) was reported in active phase of sJIA2). Therefore, serum IL-18 levels were monitored in order to find the way how to taper and discontinue TCZ in sJIA patients.
Background/Purpose: Tocilizumab (TCZ), anti-human interleukin-6 receptor monoclonal antibody, was the first biologic agent used in the treatment of systemic juvenile idiopathic arthritis (sJIA) in Japan. We have previously reported that long-term TCZ therapy could induce drug-free remission in sJIA patients resistant to conventional prednisolone (PSL) therapy1). On the other hand, extremely high levels of serum IL-18 (>10,000 pg/ml) was reported in active phase of sJIA2). Therefore, serum IL-18 levels were monitored in order to find the way how to taper and discontinue TCZ in sJIA patients.
Methods: sJIA patients treated with 8 mg/kg of TCZ for more than 1 year who were initially resistant to 3 times of consecutive weekly methyl-PSL pulse therapy in active phase and/or had been refractory to long-term oral steroid therapy were recruited. Our tapering protocol for patients who maintained clinical remission by TCZ was as following; decrease PSL dose gradually to less than 0.2mg/kg/day, prolongation of TCZ interval from every 2w to 3w, discontinuation of PSL, and discontinuation of TCZ, in tern. Serum levels of IL-6/IL-18 were measured by ELISA .
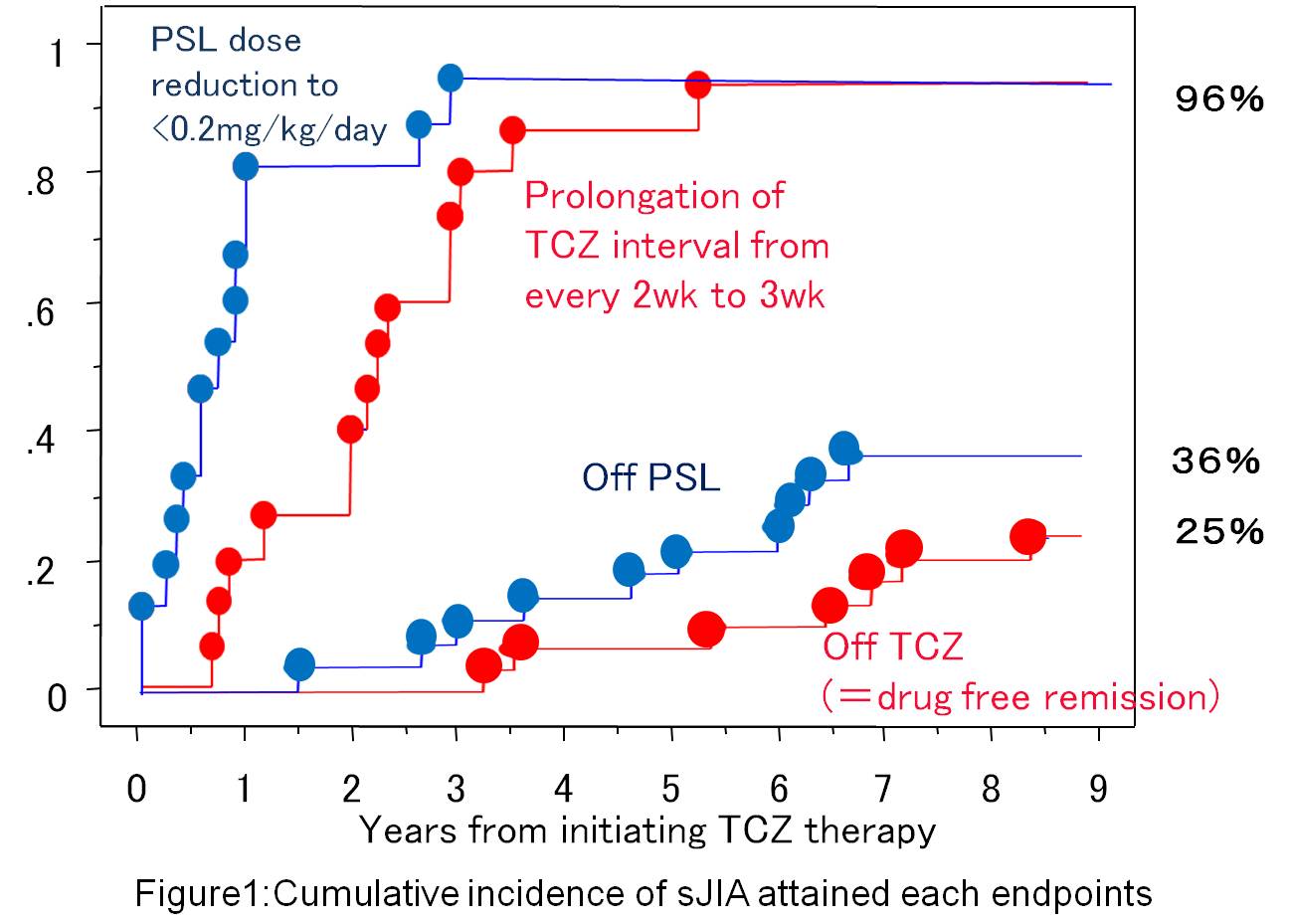
Results: A total of 28 sJIA patients were recruited in this study; their mean disease duration was 4.1 years, and mean PSL dose was 0.5mg/kg/day at initiating TCZ.
Twenty-seven patients (96%) attained clinical remission with combination therapy of PSL<0.2mg/kg/day and TCZ every 3w. Thereafter, 10 patients (36%) completed PSL-free remission, and 7 (25%) attained drug-free remission by discontinued TCZ (Figure 1). No significant difference was found in TCZ treatment period between the two patient groups who achieved the drug-free remission (mean 7.0 yrs) and who maintained clinical remission (mean 5.5 yrs).
 Serum IL-6 decreased quickly in correlating with improvement of clinical symptoms after starting TCZ. On the contrary, serum IL-18 did not decrease in a patient who was resistant to TCZ therapy, or in patients who flared or developed to macrophage activation syndrome during the TCZ therapy. In patients who attained clinical remission by TCZ, however, IL-18 steadily decreased with treatment course (figure 2). In all patients maintained the drug-free remission for more than 2 years, serum IL-18 was < 1,000 pg/ml when the TCZ was discontinued.
Serum IL-6 decreased quickly in correlating with improvement of clinical symptoms after starting TCZ. On the contrary, serum IL-18 did not decrease in a patient who was resistant to TCZ therapy, or in patients who flared or developed to macrophage activation syndrome during the TCZ therapy. In patients who attained clinical remission by TCZ, however, IL-18 steadily decreased with treatment course (figure 2). In all patients maintained the drug-free remission for more than 2 years, serum IL-18 was < 1,000 pg/ml when the TCZ was discontinued.
Conclusion: TCZ had the potential to induce drug-free remission in 1/4 of sJIA patients who had been refractory to the conventional therapy. Serum IL-18 level could be a useful bio-marker in tapering the TCZ therapy in sJIA.
1) Kubota T, et al: #267, 2011 ACR meeting
2) Shimizu M, et al
Disclosure:
T. Kubota,
None;
S. Takei,
Chugai,Eisai, Takeda, Bristol-Myers,
2,
Caugai, Eisai, Takeda, Abbvie, Astellas, Teijin, Novartis, Pfizer, Asahi Kasei,
8;
Y. Yamasaki,
None;
J. Yasumura,
None;
N. Kuwada,
None;
Y. Nonaka,
None;
T. Takezaki,
None;
T. Yamatou,
None;
T. Nagakura,
None;
Y. Nerome,
None;
H. Imanaka,
None;
H. Akaike,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/changes-in-serum-il-18-level-in-systemic-juvenile-idiopathic-arthritis-patients-who-attained-drug-free-remission-by-tocilizumab/
