Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Combination therapy of tumor necrosis factor inhibitors (TNFi) and methotrexate (MTX) is a well-established treatment that has dramatically changed outcomes in Juvenile Idiopathic Arthritis (JIA). Given the high relapse rate of patients with JIA, it is crucial to determine the appropriate timing and method of tapering medications once disease control is achieved.
Methods: This is a retrospective single-center observational cohort study of patients with polyarticular JIA (pJIA) seen in our institution’s pediatric rheumatology clinic between January 2000 and December 2011. Demographic information (Table 1), diagnosis, antibody status, medication history, and dates of achieving inactive disease and flare were extracted from patient charts. Inactive disease was defined per Wallace criteria and flare was defined as no longer fulfilling these criteria for more than 1 visit. We performed a flare-free survival analysis, looking at 4 treatment arms of patients who had achieved inactive disease and tapered medications: TNFi plus MTX, tapered off MTX first (group 1,n=45), TNFi plus MTX, tapered off TNFi first (group 2,n=19), MTX monotherapy (group 3,n=32), and TNFi monotherapy(group 4,n=6). We also evaluated outcomes based on rheumatoid factor (RF) status.
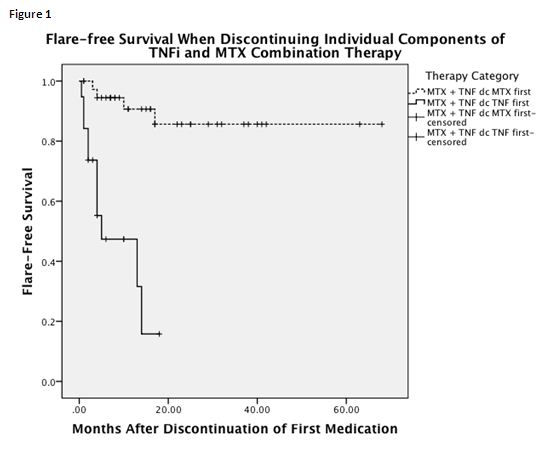
Results: We included 194 pJIA patients with a mean follow-up of 4.74 ± 2.8 years. After a mean of 23.6 months (range 5-120), 129/194 patients (66%) achieved inactive disease. Group 2 had a significantly worse outcome with only 47% of patients remaining flare-free for 12 months off TNFi despite continued MTX therapy compared to 90% of those who had weaned MTX first and continued TNFi (p<.0005) (Figure 1). After a mean of 21months (range 0-81) with inactive disease, 30% of patients were able to discontinue all medications. Of these, 33% flared within 3 months, 46% within 6 months, and 67% within 12 months. When comparing the 4 treatment arms, group 3 had a significantly better outcome in terms of flare-free survival off medications compared to group 1(p=0.007). Time to flare was independent of time in remission and RF status.
Conclusion: This study confirms that flare rates in JIA patients are high. However, when tapering TNFi/MTX combination therapy to monotherapy, weaning TNFi first carries a significantly higher risk of flare than weaning MTX first. After discontinuation of all medications, patients on MTX monotherapy had better flare-free survival compared to those on combination therapy, possibly due to inherently milder disease.
Disclosure:
C. Y. Chang,
None;
R. Meyer,
None;
A. Reiff,
Abbott Immunology Pharmaceuticals, Amgen, Genentech, Novartis, Pfizer, Roche,
5,
Abbott Immunology Pharmaceuticals, Amgen, Genentech, Novartis, Pfizer, Roche,
8.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rates-of-flare-free-survival-in-juvenile-idiopathic-arthritis-when-tapering-individual-components-of-tumor-necrosis-factor-inhibitor-and-methotrexate-combination-therapy/