Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Although methotrexate (MTX) is the cornerstone of RA treatment, use of oral and subcutaneous (SC) preparations in real-world settings is not well characterized.
Methods:
Using national data from the U.S. Medicare program 2006-2010, we identified RA patients (pts) on the basis of 2 physician diagnosis codes initiating oral MTX (no prior use ever, minimum of 6m clean period) who had no use of hydroxychloroquine (HCQ), sulfasalazine (SSZ), or leflunomide (LEF) in the previous 6 mo with no prior biologic use. During follow-up (>=1 year required), dose escalation of oral MTX and switch to SC MTX, HCQ, SSZ, and LEF were characterized. Persistence with each was examined and defined as a gap > 90 days after the end of the days’ supply. Subsequent use and timing of addition of biologics was examined among those who had at least one switch, adjusting for multiple potential confounders using Cox proportional hazards models, using the switch date as the time axis.
Results:
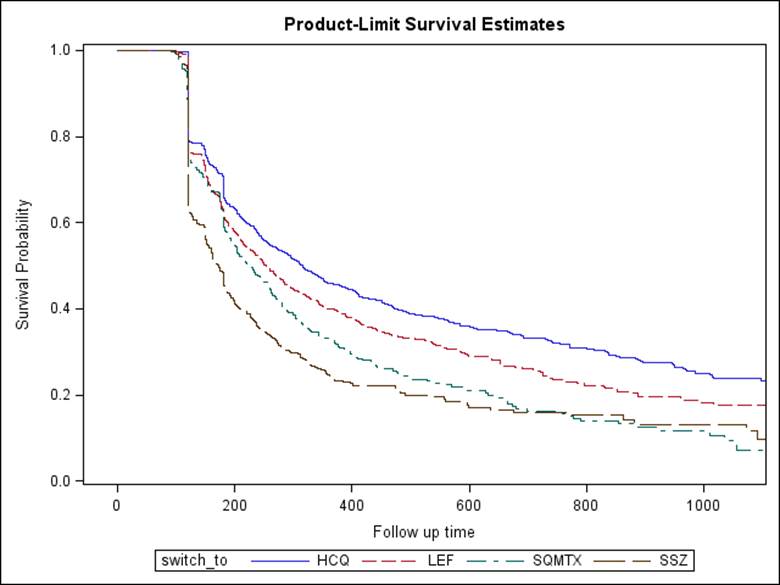
New oral MTX users (n=20406) were 76.9% women, had mean +- SD age of 69.7 +- 11.7 years and contributed a median (IQR) follow-up of 2.6 (1.7, 3.5) years; 32.1% never changed dose of oral MTX and remained at their starting dose (most common dose: 7.5mg, 22%; and 10.0mg, 23%). Of all patients, only 39.4% used oral doses of >= 20mg/week at any time. At 1 year and beyond, 75.8% stayed on oral MTX (with or without dose increase) and did not add or switch to HCQ, SSZ, LEF, or SC MTX. The remainder switched or added HCQ (11.9%), SSZ (5.0%), LEF (9.3%) or switched to SC MTX (3.9%). The median (IQR) dose of oral MTX prior to switch to SC was 15.0 (12.5, 20.0) mg/wk and occurred at a median (IQR) of 292 (133,557) days. Persistence with SC MTZ, HCQ, SSZ, and LEF was similar (figure) and was lower than with oral MTX.
Overall, 19.1% of the cohort starting oral MTX initiated a biologic, mostly (84.7%) anti-TNF therapy. Of these individuals, 55.5% had never used MTX at a dose of >= 20mg prior to biologic use; 32.8% never used MTX at a dose of >= 15mg. Among pts who used biologics after a switch to SC MTX, the median (IQR) interval of time between switching to SC MTX and initiation of a biologic was 165 (66, 336) days. Among patients who increased oral MTX dose or switched to SC MTX or other nbDMARDs (n=12,652) and after multivariable adjustment, there were no significant differences in use of biologics between patients who switched to SC MTX vs. added or switched to SSZ or LEF. Pts who added HCQ were less likely to require biologics (hazard ratio 0.53, 95% CI 0.37-0.77), with some evidence suggesting that HCQ use was a proxy for less severe RA (not shown).
Conclusion:
Titration to higher doses of oral MTX and use of SC MTX preparations among Medicare pts with RA is infrequent and may be underutilized. Further work to optimize MTX dosing before pts are switched to a biologic may be warranted.
Disclosure:
J. R. Curtis,
Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, Crescendo, AbbVie,
2,
Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, Crescnedo, AbbVie,
5;
F. Xie,
None;
J. Zhang,
Roche/Genentech,
2;
L. Chen,
None;
H. Yun,
None;
M. H. Schiff,
Antares biopharma, Abbvie, BMS, Pfizer, Amgen, Roche, UCB,
5;
D. Mackey,
None;
S. Ginsberg,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patterns-of-use-of-oral-and-subcutaneous-methotrexate-use-in-rheumatoid-arthritis-patients-enrolled-in-the-u-s-medicare-program/