Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Certolizumab pegol (CZP) is a PEGylated Fc-free anti-TNF approved in 45 countries for the treatment of rheumatoid arthritis (RA) and/or Crohn’s disease (CD). An earlier analysis of pregnancies reported to the UCB Pharma global safety database1 suggested pregnancy outcomes after maternal CZP exposure are similar to those reported for the US general population (65% live births, 17% fetal losses and 18% elective terminations; data from 6,578,000 pregnancies, 1990-2008).2 The aim was to provide an updated analysis of pregnancy outcomes after CZP exposure by including new reports and ongoing pregnancies since the last retrospective analysis.1
Methods:
The UCB Pharma global safety database was searched retrospectively for all medically confirmed cases of pregnancy, including both clinical trial (incidental exposures as all trial protocols exclude pregnant women and those not receiving reliable contraception) and post-marketing reports, through March 28 2013. The number of live births, spontaneous miscarriages and elective terminations for neonates exposed to CZP (maternal and paternal exposure) before and during pregnancy was examined. Congenital abnormalities, neonatal deaths and maternal demographics were also investigated.
Results:
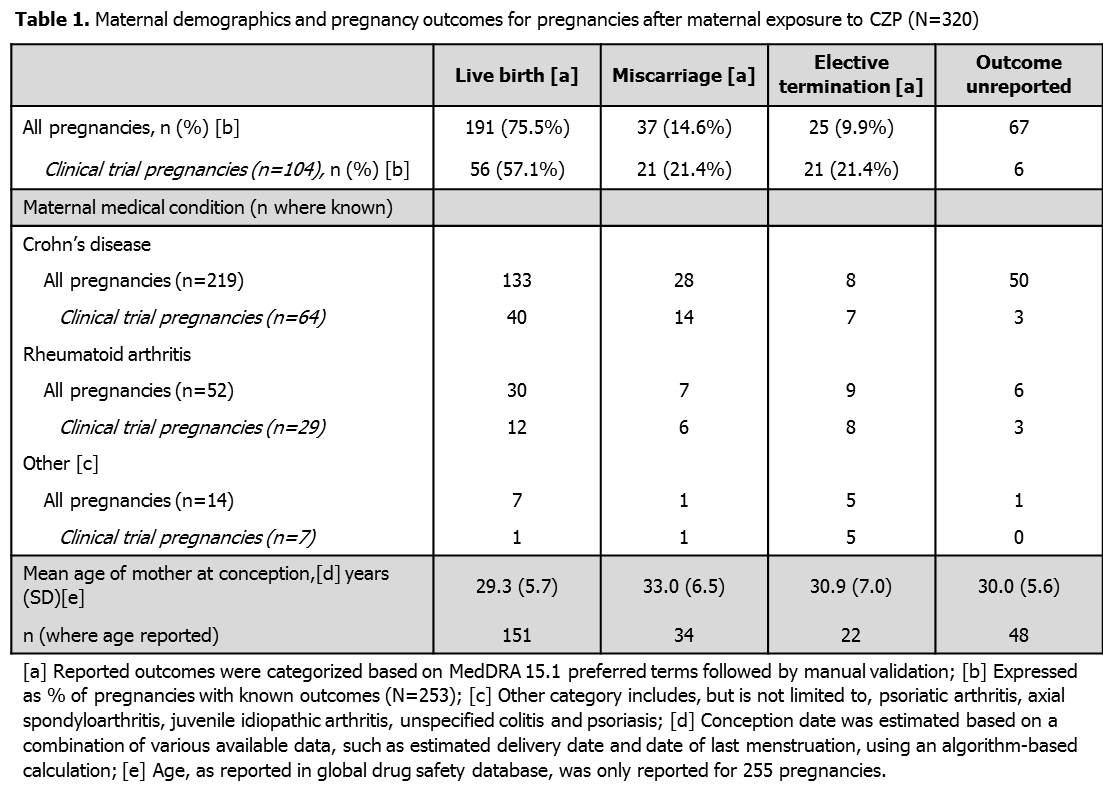
As of March 28 2013, 337 pregnancies were reported: 17/337 (5.0%) occurred following paternal exposure and 320/337 (95.0%) occurred following maternal exposure to CZP. Within the 320 maternal exposure pregnancies, 234 were reported from the US and 86 from the rest of the world. The underlying maternal conditions were CD (219/320) and RA (52/320), and the majority of mothers were <35 years old (192/320). For pregnancies following maternal CZP exposure (Table 1), outcomes were unreported for 67/320 (20.9%) and known outcomes were available for 253/320 (79.1%): 191/253 (75.5%) resulted in live births, 37/253 (14.6%) in spontaneous miscarriages and 25/253 (9.9%) women had elective terminations. Within the 191 live births after maternal CZP exposure there were 3 reported cases of congenital disorder: one infant had vesicoureteric reflux, one infant was born with congenital morbus hirschsprung and club feet, and one infant had a high aortic arch with aberrant left subclavian. None of the treating physicians considered these events related to CZP administration. A single neonatal death was reported after maternal exposure in one of a set of twins delivered before 26 weeks gestation.
Conclusion:
Updated analysis of pregnancy outcomes after exposure to CZP supported previous reports1 suggesting no obvious/apparent impact of maternal CZP exposure on pregnancy outcomes. Additional data from large numbers of pregnant women are required to fully evaluate the safety and tolerability of CZP in pregnancy.
References:
1. Clowse M. Arthritis Rheum 2012;64(Suppl10):S702; 2. Ventura S.J. Natl Vital Stat Rep 2012;60:1-21
Disclosure:
M. E. B. Clowse,
UCB Pharma,
5;
D. C. Wolf,
Abbott, Genentech, GIVEN Imaging, Janssen Biotech Inc., Millennium Research Group, Prometheus Laboratories, Salix Pharmaceuticals, UCB Pharma,
5,
Abbott, Bristol-Myers Squibb, Genentech, GIVEN Imaging, Janssen Biotech Inc., Millennium Research Group, Prometheus Laboratories, UCB Pharma,
2,
Abbott, Janssen Biotech Inc., Prometheus, Salix Pharmaceuticals, UCB Pharma, Warner Chilcott,
8;
F. Förger,
Roche Pharmaceuticals, UCB Pharma,
5,
Roche Pharmaceuticals, UCB Pharma,
8;
J. J. Cush,
Pfizer, Celgene, CORRONA, Amgen, NIH, Novartis, UCB Pharma,
2;
C. Stach,
UCB Pharma,
1,
UCB Pharma,
3;
G. Kosutic,
UCB Pharma,
1,
UCB Pharma,
3;
S. Williams,
UCB Pharma,
1,
UCB Pharma,
3;
C. Maduka,
UCB Pharma,
3;
U. Mahadevan,
Abbott, Janssen, Elan, Genentech, Shire, UCB Pharma,
5,
Prometheus, Millenium, GSK,
2.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/retrospective-analysis-of-certolizumab-pegol-use-during-pregnancy-update-of-impact-on-birth-outcomes/