Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The efficacy and safety of subcutaneous tocilizumab (TCZ SC) were demonstrated in a 24 week (wk) randomized clinical trial (SUMMACTA). The cumulative safety, immunogenicity and continued efficacy in patients (pts) with RA who were rerandomized to receive either TCZ SC or TCZ IV in the open label (OL) period was evaluated at 49 wks.
Methods: SUMMACTA is a 2-year, Phase 3 trial, which is a randomized, active controlled, parallel group study that includes a 24 wk double-blind (DB) period, followed by a 72 wk OL phase. During the DB period, pts were randomized 1:1 to receive TCZ SC weekly (qw) or TCZ IV every 4 wks (q4w) in combination with traditional DMARDs. After the DB period, pts who initially received TCZ SC 162 mg qw were re-randomized 11:1 to receive TCZ SC 162 mg qw or TCZ IV 8mg/kg q4w and pts who initially received TCZ IV 8mg/kg q4w were re-randomized 2:1 to receive TCZ IV 8mg/kg q4w or TCZ SC 162 mg qw.
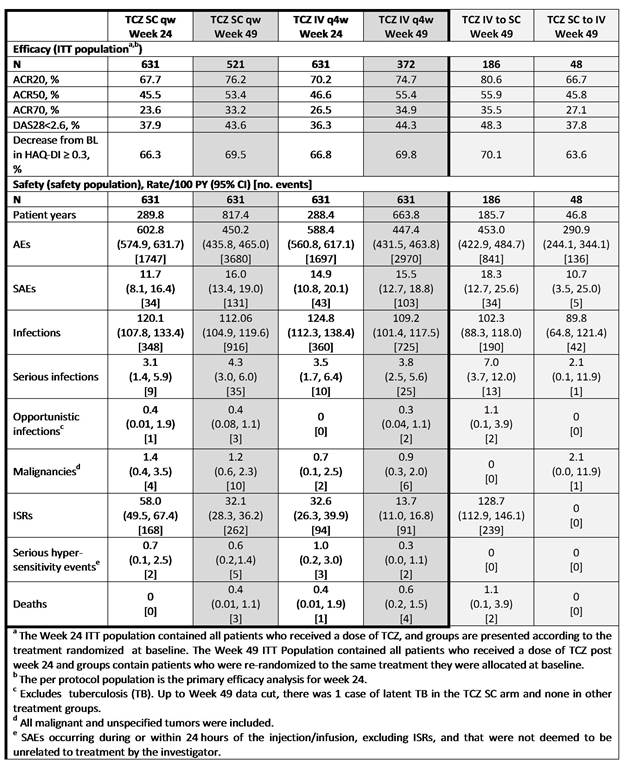
Results: A total of 1262 pts were initially enrolled (TCZ SC, n = 631; TCZ IV, n = 631). At Wk 25, 521 pts continued TCZ SC and 372 pts continued TCZ IV; 48 pts switched from TCZ SC to TCZ IV and 186 pts switched from TCZ IV to TCZ SC. 61 (12%) pts in the TCZ SC group and 43 (12%) pts in the TCZ IV group withdrew from the study in the OL period; most commonly due to safety. The percentage of pts who continued TCZ SC and TCZ IV who achieved ACR20/50/70 responses, DAS28 remission and an improvement from baseline in HAQ-DI ≥ 0.3 were sustained up to 49 wks and were comparable between the groups (Table). Efficacy in pts after switching from TCZ IV to TCZ SC or TCZ SC to TCZ IV was comparable to pts who received continuous TCZ SC or TCZ IV. Rates of adverse events (AE), serious AEs, serious infections and malignancies for TCZ SC and TCZ IV were comparable between 24 and 49 wks (Table). The rate of injection site reactions (ISRs) decreased over time, but remained higher for pts receiving TCZ SC compared with pts receiving TCZ IV, as previously reported for wk 24. There were 3 deaths each in the TCZ SC and TCZ IV groups that occurred between wks 24 to 49. AntiTCZ antibody development remained low to wk 48 and no anaphylaxis occurred. The safety profile of pts who switched from TCZ IV to TCZ SC and vice versa were similar to pts who received continuous TCZ SC or TCZ IV.
Conclusion: These data demonstrate that efficacy rates for pts continuing on TCZ SC over 49 wks are maintained and remain comparable to TCZ IV, as shown at Wk 24. The wk 49 cumulative safety profiles of pts on TCZ SC were consistent with Wk 24 data and to TCZ IV, with the exception of ISRs, which were lower in the TCZ IV group. The efficacy and safety profile of pts who switched from TCZ IV to TCZ SC and vice versa was similar to pts who remained on TCZ SC or TCZ IV. AntiTCZ antibody (including IgE isotype) development did not correlate with AEs or loss of efficacy with prolonged TCZ SC exposure. TCZ SC formulation could provide an additional, more convenient administration route for pts with RA.
Disclosure:
G. Burmester,
Roche, Abbott, Pfzer, UCB, Merck Sharp and Dohme and Bristol-Myers Squibb,
2,
Roche, Chugai, Pfizer, UCB and Bristol-Myers Squibb,
5,
Roche, Pfizer, Merck Sharp and Dohme, Abbott and Bristol-Myers Squibb,
8;
A. Rubbert-Roth,
Roche and Pfizer,
2,
Roche, Chugai, Pfizer, UCB and Merck Sharp and Dohme,
5,
Roche and UCB,
8;
A. G. Cantagrel,
UCB and Pfizer ,
2,
Roche, Chugai, Pfizer, UCB, Abbott and Bristol-Myers Squibb,
5;
S. Hall,
None;
P. Leszczynski,
Roche Pharmaceuticals,
5;
D. Feldman,
None;
M. J. Rangaraj,
Roche Pharmaceuticals,
2;
G. Roane,
None;
C. L. Ludivico,
None;
E. F. Mysler,
Roche ,
2,
Roche,
5,
Roche ,
8;
C. Wells,
Roche Products Ltd. ,
3;
M. Bennett,
Roche Products Limited,
3;
I. Vranic,
Roche Products Limited ,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-efficacy-and-safety-of-tocilizumab-subcutaneous-versus-tocilizumab-intravenous-in-combination-with-traditional-dmards-in-patients-with-ra-at-49-weeks-summacta/