Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Retreatment with rituximab (RTX) is common clinical practice, although several aspects regarding retreatment, such as frequency, need to be further elucidated. The aim of this study was to describe the effectiveness of repeated courses of RTX in a large observational cohort of real-life patients with RA.
Methods: Pooled data from the Collaborating European Registries for Rituximab in RA (CERERRA) project were used. Patients with RA who received at least 4 cycles with RTX were identified and included in the analysis. A covariate-adjusted mixed effects model was fitted to the longitudinal DAS28 for patients with complete covariate information, including country, sex, age, anti-CCP status, number of prior biologics and concomitant DMARD treatment.
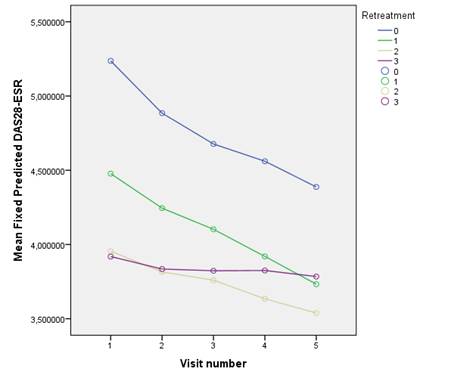
Results: 340 patients met the eligibility criteria for these analyses. At baseline (start of RTX) the mean (SD) age was 53.5 (12.6) years and the mean (SD) disease duration was 12.1 (8.2) years. Patients were 83% females, 79% RF positive and 74% anti-CCP positive. 60% were treated with corticosteroids and 84% with concomitant DMARD. Patients had failed 2.7 (1.5) prior DMARDs and 1.1 (1.0) prior biologic agents. The baseline DAS28 was 6.0 (1.4). In figure 1 the mean fixed predicted decrease of DAS28 after the first cycle and after each retreatment (1st, 2nd and 3rd) is shown. Significant improvement in DAS28 (p<0.001) was observed for each course of RTX. Comparison between curves (based on estimated marginal means) revealed significant difference between the first cycle (0) and the fourth (3rd retreatment), p<0.0001, and borderline significant difference between first cycle and 2nd retreatment (p=0.05).
 |
Conclusion: Repeated retreatment with RTX can lead to further clinical improvement after the first course of RTX.
Disclosure:
K. Chatzidionysiou,
None;
E. Lie,
Pfizer Inc,
5,
Roche Pharmaceuticals,
5,
Abbott Immunology Pharmaceuticals,
5,
Bristol-Myers Squibb,
5;
E. Nasonov,
None;
G. Lukina,
None;
M. L. Hetland,
None;
U. Tarp,
None;
K. Pavelka,
Roche, BMS, Pfizer, MSD, AbbVie,
8;
C. Gabay,
Roche Pharmaceuticals,
2,
Roche, Abbvie, Pfizer, UCB, BMS, MSD,
5;
D. Nordström,
None;
H. Canhao,
None;
M. Tomsic,
None;
P. van Riel,
None;
J. Gomez-Reino,
None;
I. Ancuta,
None;
T. Kvien,
None;
R. van Vollenhoven,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-repeated-courses-of-rituximab-in-ra-results-from-the-cererra-collaboration/
