Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Pregabalin is often administered for treatment of fibromyalgia (FM) at daily doses lower than approved by the US Food and Drug Administration (starting dose 150 mg; recommended dose 300–450 mg). The objective of this post-hoc analysis was to characterize pregabalin efficacy across a range of doses and set expectations regarding the incidence of adverse events (AEs) through the course of FM treatment.
Methods: A hyperbolic Emax dose-response model using patient data pooled from 3 FM placebo-controlled trials examined the dose-response of pregabalin for pain (≥30% pain response), patient global impression of change (PGIC) and sleep quality. All patients had a diagnosis of FM based on the 1990 ACR criteria. After starting treatment, new incidences of AEs by study week were used to assess safety. Trials are identified by Pfizer study number (ClinicalTrials.gov identifier): 1008-105, A0081056 (NCT00645398), A0081077 (NCT00230776).
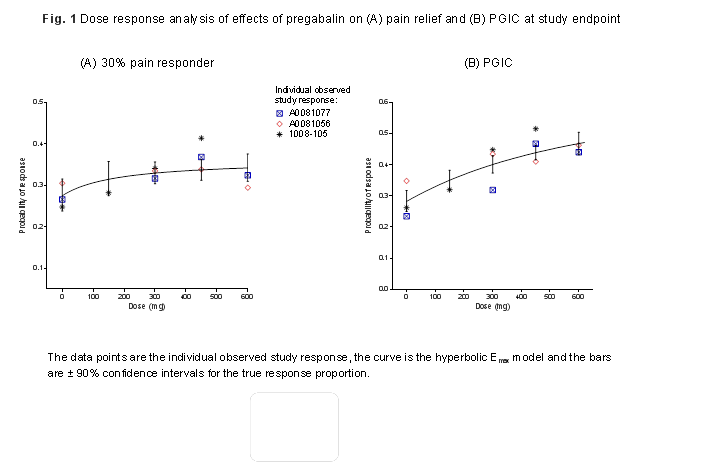
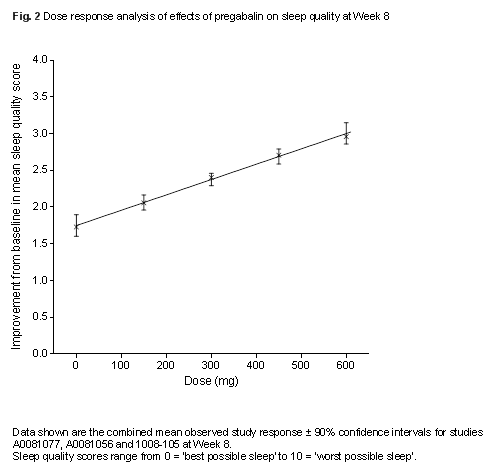
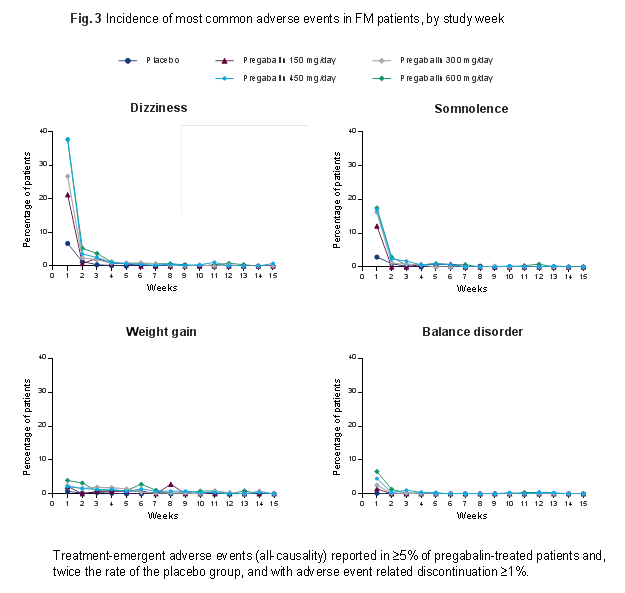
Results: The likelihood of FM patients achieving ≥30% pain response incrementally increased from 27.5% (90% CI, 23.8–31.5%) with placebo to 31.5% (27.5–35.8%) at 150 mg/d, 33.0% (30.4–35.7%) at 300 mg/d, 33.7% (31.3–36.3%) at 450 mg/d and 34.2% (31.0–37.6%) at 600 mg/d. The likelihood of improvements in PGIC increased in a dose-dependent manner with higher pregabalin doses (Fig. 1). Incremental improvements in sleep quality also occurred with increasing doses (Fig. 2). It was not possible to estimate the value of Emax and ED50 parameters for sleep quality over the current dose range and therefore the upper plateau of the dose-response curve was not attained for this endpoint. The resulting curve appears linear, a finding not expected over a broader dose range or using data from different trials. Dizziness and somnolence were commonly reported AEs. New incidences of dizziness and somnolence were highest after 1 week of treatment and were considerably fewer subsequently, decreasing week by week for the same dose (Fig. 3).
Conclusion: These data demonstrate the dose-response of pregabalin for pain, PGIC, and sleep, and highlight the incremental benefit of achieving the maximum recommended doses of 300–450 mg/d for treatment of FM. Common AEs are generally seen within 1 week of starting treatment, with few subsequent new reports for the same dose. This study was sponsored by Pfizer.
To cite this abstract in AMA style:
Clair A, Whalen E, Thomas N, Pauer L. Pregabalin Dose-Response for Sleep Quality and Pain Response in Fibromyalgia: A Post-Hoc Analysis of Three Randomized Trials [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/pregabalin-dose-response-for-sleep-quality-and-pain-response-in-fibromyalgia-a-post-hoc-analysis-of-three-randomized-trials/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pregabalin-dose-response-for-sleep-quality-and-pain-response-in-fibromyalgia-a-post-hoc-analysis-of-three-randomized-trials/