Apremilast for the Treatment of Behçet’s Syndrome: A Phase II Randomized, Placebo-Controlled, Double-Blind Study
Background/Purpose: Mucocutaneous manifestations of Behçet’s syndrome (BS) can be resistant to conventional treatment and at times disabling. Apremilast (APR), an oral phosphodiesterase 4 inhibitor, modulates inflammatory pathways. This study investigated the efficacy of APR in BS patients with active oral ulcers (OU).
Methods: This was a phase II, multicenter, randomized, placebo (PBO)-controlled study with an open-label extension. Patients with BS without major organ involvement but ≥2 OU were randomized to APR 30 mg BID or PBO for 12 weeks followed by a 12-week active-treatment period for all patients and a 28-day post-treatment observational follow-up. The primary endpoint was the number of OU at week 12. Secondary endpoints were the number of genital ulcers (GU) at week 12, efficacy over time (AUC for OU and GU, first 12 weeks), disease activity (BSAS, BDCAF), patient-reported outcomes (BDQOL, SF-36), and adverse events (AEs).
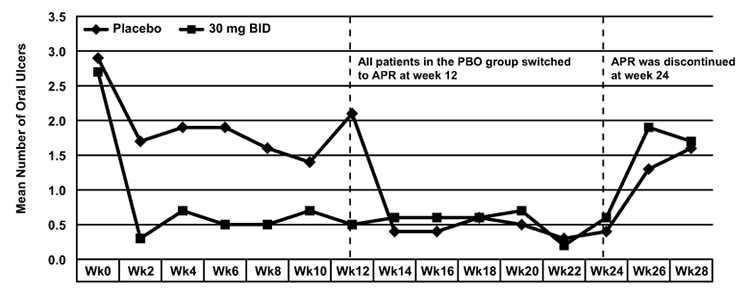
Results: 111 patients (mean age 34.5 ± 10.1 years, 69% women) were randomized (55 APR, 56 PBO) and 95 completed the treatment phase (50 APR, 45 PBO). Mean (±SD) number of OU at week 12 was 0.5 ± 1.03 with APR and 2.1 ± 2.58 with PBO (P<0.0001). The figure shows the mean number of OU over time. Notably, the beneficial effect of APR on OU started within 2 weeks. There was a sustained effect while on APR, but the effect disappeared shortly after APR was stopped at week 24. Improvement in OU pain (VAS) was significantly higher with APR (-44.7 ± 24.30 vs -16.0 ± 32.54; P<0.0001). At week 12, significantly more patients receiving APR had a complete response (OU-free) (71% [39/55], APR; 29% [16/56], PBO; P<0.0001). Among the limited number of patients with GU at baseline (n=16), 10/10 receiving APR had a complete response at week 12 vs 3/6 receiving PBO (p=0.036). The mean change from baseline in the BDCAF, BSAS and BDQOL scores were significantly higher in the APR group (APR vs PBO -1.5 ± 1.84 vs -0.1 ± 1.51; P=0.0007, -21.2 ± 17.9 vs -5.9 ± 18.2; p<0.0001 and -4.5 ± 7.61 vs -1.6 ± 5.3 P=0.0397 respectively). There were 2 serious AEs with APR (1 diplegia, 1 anal fissure and hemorrhoids) and 3 with PBO (2 disease flares, 1 fever episode). The diplegia in 1 patient was transient and was not thought to be related to APR.
Conclusion: APR was effective in the treatment of OU, the cardinal manifestation of BS, with an acceptable safety profile. There were also indications of efficacy in several secondary endpoints, including GU, a highly specific lesion for BS. Further trials are warranted to test the efficacy of APR for other manifestations of BS.
Disclosure:
G. Hatemi,
None;
M. Melikoglu,
None;
R. Tunc,
None;
C. Korkmaz,
None;
B. Turgut Ozturk,
None;
C. Mat,
None;
P. A. Merkel,
Celgene Corporation,
2;
K. Calamia,
None;
Z. Liu,
Celgene Corporation,
3;
L. Pineda,
Celgene Corporation,
1,
Celgene Corporation,
3;
R. M. Stevens,
Celgene Corporation,
1,
Celgene Corporation,
3;
H. Yazici,
None;
Y. Yazici,
Celgene Corporation,
2,
Celgene Corporation,
5.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/apremilast-for-the-treatment-of-behcets-syndrome-a-phase-ii-randomized-placebo-controlled-double-blind-study/