Session Information
Session Type: Late-Breaking Abstract Session
Session Time: 8:15AM-8:30AM
Background/Purpose: Deucravacitinib is an oral, selective tyrosine kinase 2 (TYK2) inhibitor being investigated in active PsA in the global, randomized, double-blind, placebo (PBO)-controlled, phase 3 POETYK PsA-1 (NCT04908202) and PsA-2 (NCT04908189) studies. Deucravacitinib has an established clinical profile in moderate to severe plaque psoriasis (PsO), with over 5 years of data, and is approved in multiple countries for PsO. We report efficacy and safety of deucravacitinib up to W52 in POETYK PsA-1.
Methods: Patients had a PsA diagnosis for ≥ 3 mo, met CASPAR criteria, were naive to biologic DMARDs, and had active or documented PsO, active arthritis (≥ 3 swollen and ≥ 3 tender joints), a high-sensitivity CRP level of ≥ 3 mg/L, and ≥ 1 PsA-related hand and/or foot joint erosion on radiographs at screening. Patients (N = 670) were randomized 1:1 to receive deucravacitinib 6 mg QD (n = 336) or PBO (n = 334) through W16. From W16 to W52, those randomized to PBO were switched to deucravacitinib 6 mg QD. The primary endpoint was ACR 20 at W16. Secondary endpoints included measurements of PsA disease activity, joint and extraarticular clinical responses, radiographic progression, and quality of life. Treatment discontinuation prior to W16 was considered an intercurrent event and imputed as a nonresponse. Nonresponder imputation and control-based pattern imputation were used for the remaining missing binary and continuous data, respectively. Post hoc analyses of radiographic data included all available assessments at baseline, W16, and W52, without imputation. Safety was evaluated through W52.
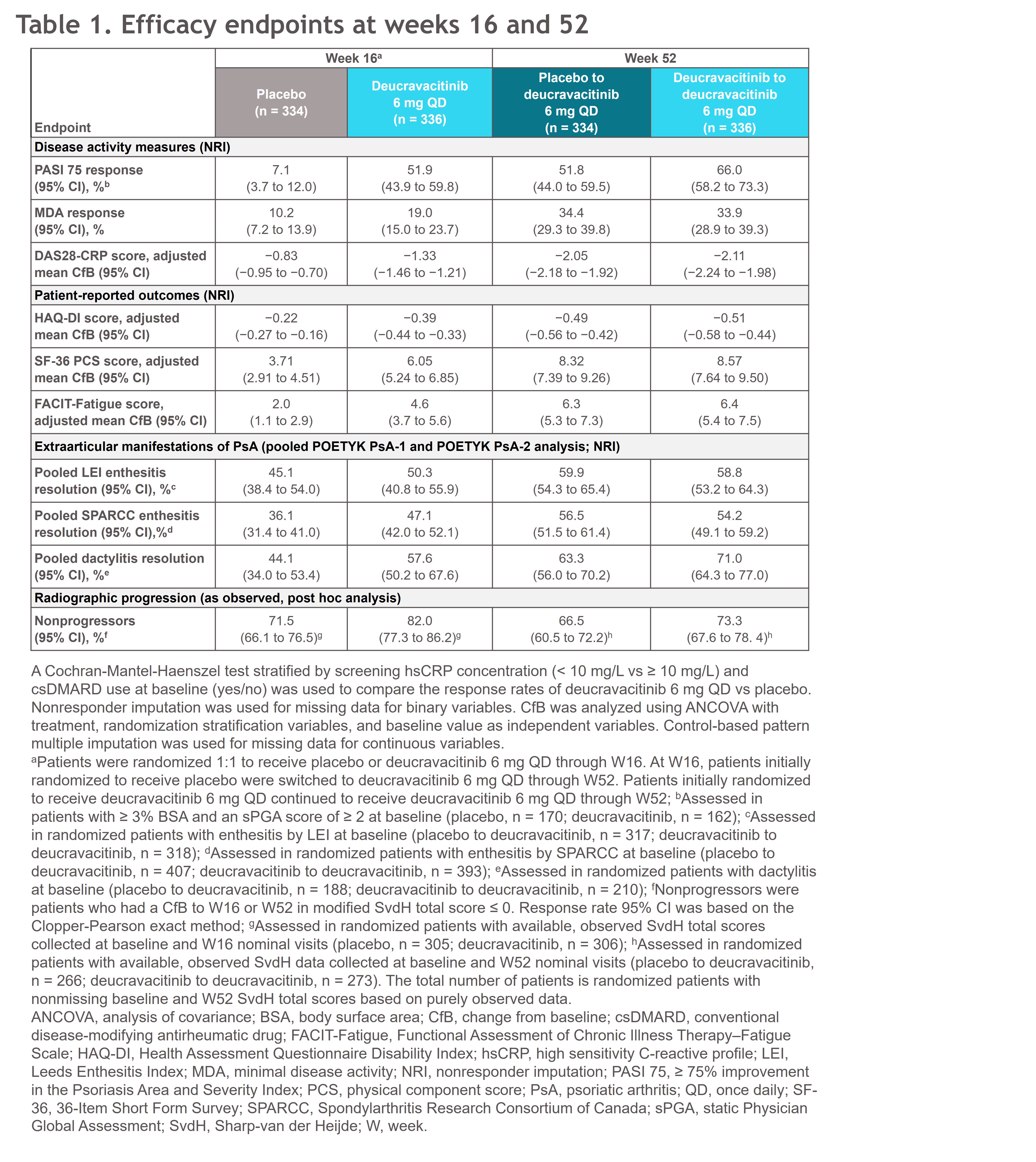
Results: Demographics and baseline characteristics were balanced across arms. The study met the primary endpoint (ACR 20; P < 0.0001).1 Responses continued to improve through W24 and were maintained with deucravacitinib through W52; similar results were seen for ACR 50/70 (Figure 1A). Clinical responses and patient-reported outcomes (PROs) were met at W16, continued to improve, and were maintained through W52 with deucravacitinib (Table 1). Inhibition of radiographic progression observed at W16 with deucravacitinib1 was sustained through W52 (Figure 1B). Patients who switched from PBO to deucravacitinib at W16 had reduced radiographic progression after switching (Figure 1B). Few serious adverse events (AEs) and discontinuations due to AEs occurred; deucravacitinib was well tolerated through W52 (Table 2). No new safety signals related to adjudicated MACE, VTE, or opportunistic infections were observed. No deaths occurred.
Conclusion: Deucravacitinib was efficacious in patients with PsA based on clinical responses, PROs, and inhibition of radiographic progression, which continued to improve after W16 and were durable through W52. Responses at W52 were comparable in patients who switched from PBO to deucravacitinib at W16. Safety was consistent with the known deucravacitinib profile, with no new signals. Data were consistent with findings from POETYK PsA-2, reinforcing deucravacitinib as efficacious in PsA and underscoring its favorable safety profile as the first selective TYK2 inhibitor evaluated in PsA.
Reference
van der Heijde D, et al. Ann Rheum Dis 2025;84(supp 1):313–314.
To cite this abstract in AMA style:
van der Heijde D, Mease P, Paul C, Behrens F, Gossec L, Kaneko Y, Eder L, Coates L, Pink A, Wan W, Soriano E, Leszczyński P, Scher J, Deodhar A, Pachai C, Li J, Fan X, Rabbat S, Sardinas C, Plewinski M, Vaile J, Merola J. Efficacy and Safety of Deucravacitinib up to Week 52: A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study in Patients With Active Psoriatic Arthritis Who Are Naive to Biologic Disease-Modifying Antirheumatic Drugs [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-deucravacitinib-up-to-week-52-a-multicenter-randomized-double-blind-placebo-controlled-phase-3-study-in-patients-with-active-psoriatic-arthritis-who-are-naive-to-biologic-d/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-deucravacitinib-up-to-week-52-a-multicenter-randomized-double-blind-placebo-controlled-phase-3-study-in-patients-with-active-psoriatic-arthritis-who-are-naive-to-biologic-d/

.jpg)
.jpg)