Session Information
Date: Tuesday, October 28, 2025
Title: Abstracts: Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science (2591–2596)
Session Type: Abstract Session
Session Time: 1:30PM-1:45PM
Background/Purpose: TVB-009 is being developed as a proposed biosimilar candidate to the reference denosumab. Biosimilarity of TVB-009 to US-licensed denosumab (US-denosumab) and EU-approved denosumab was demonstrated in healthy participants in a Phase 1 pharmacokinetic/pharmacodynamic study. No clinically relevant differences in efficacy or safety between TVB-009 and US-denosumab were demonstrated in women with postmenopausal osteoporosis (PMO) in the 52-week main treatment (tx) period of a 78-week Phase 3 study (NCT04729621). The objective of this analysis was to evaluate the impact of a single switch from US-denosumab to TVB-009 on safety, immunogenicity, and efficacy in women with PMO during the subsequent 26-week transition period.
Methods: Eligible participants included postmenopausal women aged 60–90 years with a diagnosis of osteoporosis and a lumbar spine bone mineral density (LS-BMD) measurement T-score of ≥−4.0 to < −2.5 at screening. At baseline, participants were randomized 1:1 to receive 60 mg of TVB-009 or US-denosumab by subcutaneous injection on Day 1 and at Week 26 in the main tx period. In the transition period, participants in the US-denosumab arm were re-randomized 1:1 to receive a single dose of TVB-009 or a third dose of US-denosumab at Week 52, while participants in the TVB-009 arm received a third dose of TVB-009.
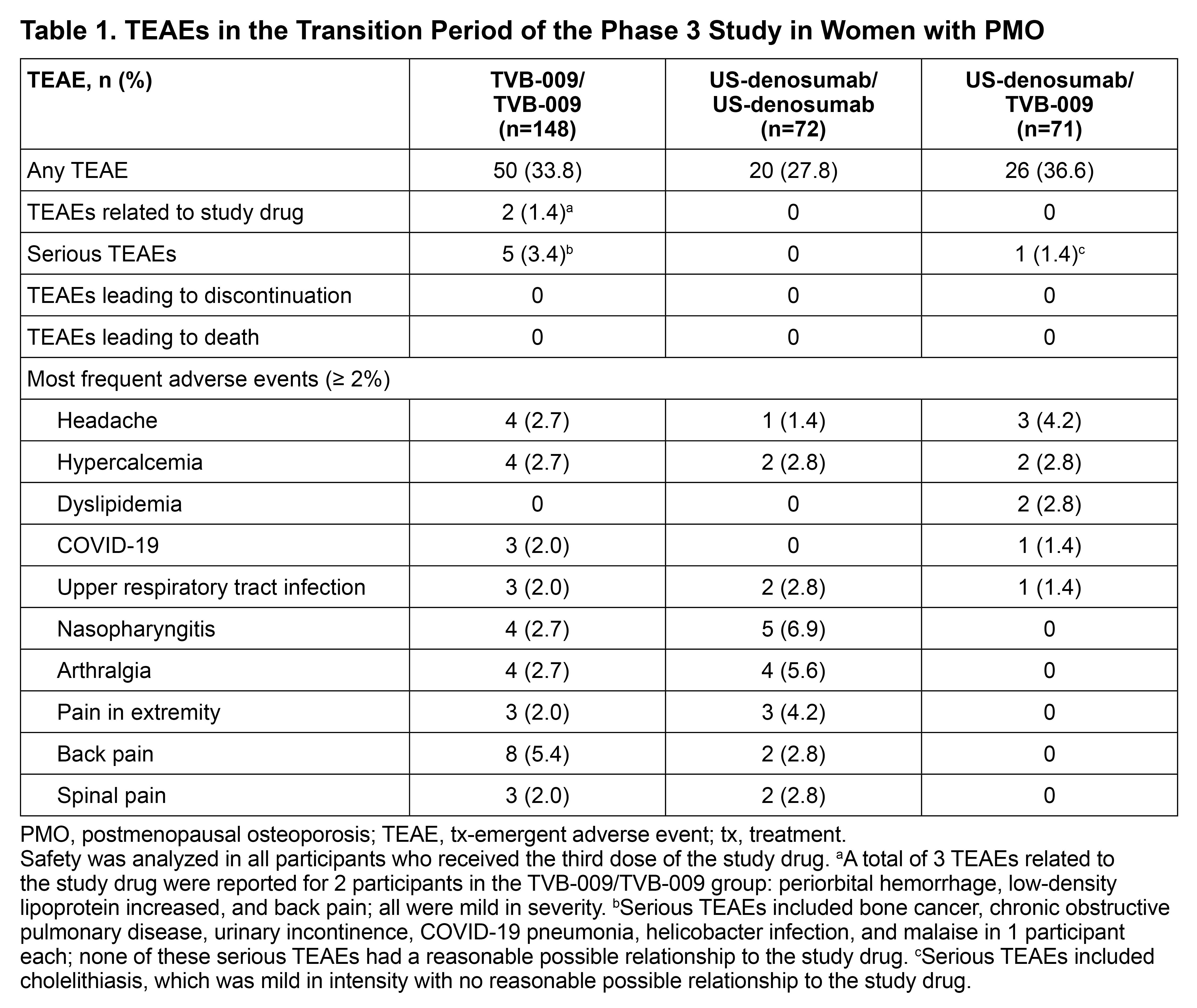
Results: Of 332 participants initially randomized, 291 completed the main tx period and were randomized in the transition period (TVB-009/TVB-009, n=148; US-denosumab/US-denosumab, n=72; US-denosumab/TVB-009, n=71). In the transition period, comparable proportions of participants experienced tx-emergent adverse events (TEAEs) across the 3 tx groups (Table 1). One participant in the US-denosumab/TVB-009 group experienced a serious TEAE of cholelithiasis, which was mild and not related to the study drug. Five (3.4%) participants in the TVB-009/TVB-009 tx group experienced serious TEAEs that were unrelated to tx; none were reported in the US-denosumab/US-denosumab group. TEAEs reported in ≥5% of participants in either tx group were back pain (5.4% vs 2.8%), nasopharyngitis (2.7% vs 6.9%), and arthralgia (2.7% vs 5.6%) in the TVB-009/TVB-009 vs US-denosumab/US-denosumab groups, respectively. Notably, no TEAEs in ≥5% of participants were reported in the US-denosumab/TVB-009 group (Table 1). Similar proportions of participants in US-denosumab/US-denosumab and US-denosumab/TVB-009 were anti-drug antibody (ADA)-positive (7.1% vs 5.7%) and ADA-positive, not tx related (5.7% vs 1.4%; Table 2). None of the participants developed hypersensitivity reactions. The mean (standard deviation) percent change in LS-BMD from Week 52 to Week 78 was comparable between the US-denosumab/US-denosumab and US-denosumab/TVB-009 groups (1.15 [3.44] and 1.24 [3.07]); similar trends were observed for femoral neck and total hip BMD.
Conclusion: Women with PMO who single-switched from reference US-denosumab to TVB-009 demonstrated a comparable safety, immunogenicity, and efficacy profile to those who remained on US-denosumab. Our results demonstrate that a single switch from reference denosumab to TVB-009 does not affect safety and efficacy in this population.
To cite this abstract in AMA style:
Pavelka K, Schneider F, S D, Timan B, Barkay H, Buchner A. Single Switch from Reference Denosumab to TVB-009 in Women with Postmenopausal Osteoporosis: Analysis of the Phase 3, Randomized, Double-Blind, Multinational, Multicenter Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/single-switch-from-reference-denosumab-to-tvb-009-in-women-with-postmenopausal-osteoporosis-analysis-of-the-phase-3-randomized-double-blind-multinational-multicenter-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/single-switch-from-reference-denosumab-to-tvb-009-in-women-with-postmenopausal-osteoporosis-analysis-of-the-phase-3-randomized-double-blind-multinational-multicenter-study/

.jpg)