Session Information
Date: Tuesday, October 28, 2025
Title: (2437–2469) Systemic Lupus Erythematosus – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) may be associated with high-risk pregnancies, especially in the presence of specific predisposing factors. While optimal disease control during pregnancy is critical, there is limited evidence on the safety of belimumab exposure in the context of pregnancy (1). This study aimed to assess maternal, embryo-fetal and neonatal outcomes in patients with SLE, exposed to belimumab during the year preceding pregnancy, according to whether treatment was continued or not at time of conception.
Methods: Data were collected from: (1) the GR2 study, a French prospective cohort of pregnancies in women with auto-immune diseases involving 76 centers, (2) the French Teratology Information Service (CRAT) and (3) a national call for observations from the French Rheumatism and Inflammatory Club (CRI). Eligible participants were pregnant women aged ≥ 18 years with SLE according to the 2012 SLICC criteria, treated with belimumab within the year prior conception. Patients were stratified based on belimumab continuation (beli+) or discontinuation (beli-) at conception. Maternal disease activity, obstetrical complications and adverse embryo-fetal and neonatal outcomes were evaluated.
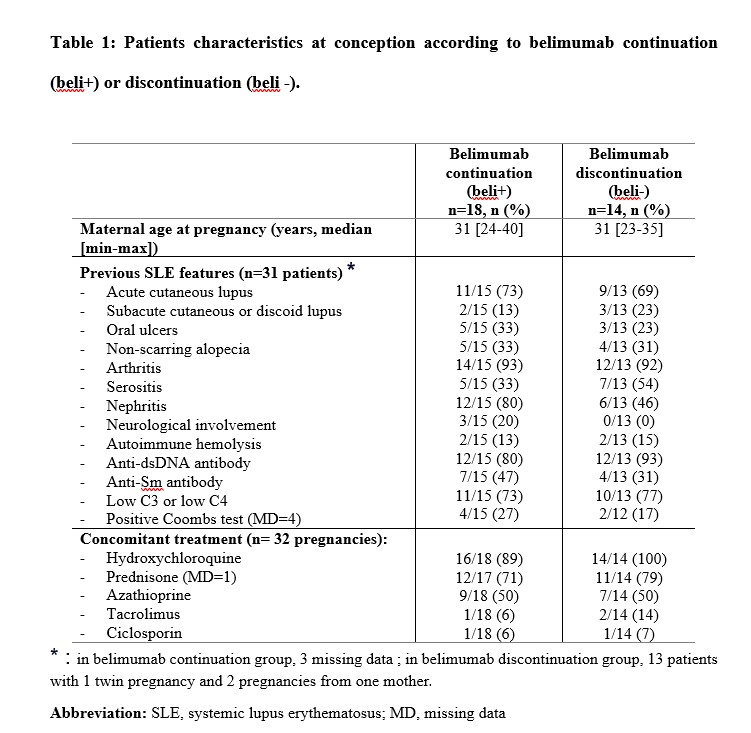
Results: A total of 32 pregnancies from 31 mothers were recorded between March 2016 and December 2024 across 19 French centers (n=19 from the GR2, n=8 from the CRAT and n=5 from the CRI) including 18 pregnancies in beli+ and 14 in beli-. Their characteristics are described in Table 1 and their exposure to belimumab in Table 2. Among the 32 pregnancies, 25 resulted in 26 live births including 14 in beli+ and 12 (1 twin pregnancy) in beli-. Pregnancy losses (n=7) included 5 miscarriages (3 in beli+ and 2 in beli-), 1 in utero fetal death due to intervillositis (beli-) and 1 voluntary abortion for non-medical reasons (beli+). Obstetrical complications occurred in 13/32 pregnancies (9 beli+, 4 beli-), including 3 maternal infections (2 beli+, 1 beli-), 2 preeclampsia (1 beli+, 1 beli-), 2 intrauterine growth restrictions (1 beli+, 1 beli-), 1 small for gestational age (beli +), 1 placenta abruption (beli-), 1 gestational diabetes (beli+), 1 postpartum hemorrhage (beli+) and/or 7 preterm deliveries (5 beli+, 2 beli-). No congenital malformations were identified. Mild to moderate lupus flares occurred during 8/32 pregnancies (25%, 4 beli+, 4 beli-), at a median gestational age of 10 weeks [5-21] and in 6 during the year post-partum (2 beli+, 4 beli-). Of the 26 live births, most neonates (n=18, 72%, 1 missing data) had normal Apgar scores. Three neonates (beli+) required hospitalization due to neonatal asphyxia (n=1) and prematurity (n=2). One neonate was suspected of congenital CMV infection (beli +). No atypical infections were reported within the first year of life of the children in either group.
Conclusion: Adverse maternal and fetal outcomes were observed in both groups, but no specific risk was identified with belimumab continuation at time of conception or during pregnancy. No malformation was reported.1. Petri M, Landy H et al. Belimumab use during pregnancy: a summary of birth defects and pregnancy loss from belimumab clinical trials, a pregnancy registry and postmarketing reports. Ann Rheum Dis. févr 2023;82(2):217‑25.
To cite this abstract in AMA style:
El Ouazzani K, Morel N, Beghin D, GUETTROT-IMBERT G, JOURDE-CHICHE N, DEROUX A, Sarrot-Reynauld F, Couzi L, Langlois V, LAZARO E, ORQUEVAUX P, DE SAINTE MARIE B, Deve K, Deligny c, Ferreira N, Godeau B, Le Guern V, Meunier R, Pasquier E, Queyrel Moranne V, Sailler L, Saint-Pastou C, Costedoat-Chalumeau N, Marin B, Richez C. Maternal, Embryo-Fetal And Neonatal Outcomes Following Belimumab Exposure During Pregnancy In Patients With Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/maternal-embryo-fetal-and-neonatal-outcomes-following-belimumab-exposure-during-pregnancy-in-patients-with-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/maternal-embryo-fetal-and-neonatal-outcomes-following-belimumab-exposure-during-pregnancy-in-patients-with-systemic-lupus-erythematosus/

.jpg)