Session Information
Date: Tuesday, October 28, 2025
Title: (2437–2469) Systemic Lupus Erythematosus – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Anifrolumab is a biologic recently approved for systemic lupus erythematosus (SLE), with efficacy shown in trials, but data from real-world practice remain limited. The aim of this registry is to evaluate the effectiveness and safety of anifrolumab at 3 and 6 months (m) in SLE patients under real-life routine care in Spanish rheumatology centers.
Methods: Multicenter, observational, ambispective study including SLE patients (2019 EULAR/ACR criteria) from 28 centers, treated with anifrolumab and followed at baseline, 3, and 6m. Clinical, lab, and treatment data were collected. Disease activity (SLEDAI-2k, SLE-DAS, PGA), damage (SLICC/ACR/DI), remission (DORIS-21), low disease activity (LLDAS), acute-phase reactants (CRP, ESR), response to treatment and medication changes were assessed. Efficacy and security comparisons across visits were done using Friedman’s test (adjusted by Holm’s method) and Cochran’s Q with McNemar’s test (Bonferroni correction) for binary outcomes.
Results: Among 133 patients, 100 completed the 3-month visit and 82 the 6-month visit. Median age was 49 years (IQR ±17), and time from diagnosis was 31 years (±16); 87.9% were female, 77.3% Caucasian and 14.4% Hispanic; 56.5% were currently employed and 19.8% were active smokers. Significant improvements at 3 and 6m compared with the baseline visit were observed in SLEDAI, SLEDAS, PGA, the percentage of patients in remission DORIS-21 and in low disease activity (LLDAS), and the number of concomitant disease modifying antirheumatic drugs, and in those receiving glucocorticoids 6m after initiation of anifrolumab vs. the basal visit (Fig. 1-2). Flare counts significantly dropped from 2 (±2) at baseline to 0 (±0 at 3m and ±1 at 6m). Response rates were: physician’s criteria 77.1% at 3m and 75.7% at 6m; 50% response in 63.5% and 65.1%; 20% response in 83.6% and 74.5%; serological response in 41.3% and 34.9% (all p = not significant) (Fig. 2). Discontinuation occurred in 5/95 patients (5%) before 3m and 10/72 (12.2%) between 3–6m. Fourteen hospital admissions (10.5%) occurred during those 6m: 5 due to SLE activity, 2 to infections, 3 to both SLE activity and infection and 4 to other reasons. Five (3.8%) patients presented a COVID-19 and 4 (3%) a herpes zoster infection since anifrolumab onset. One new case of depression and 2 new psychotropic treatments were noted. None of the patients died since starting anifrolumab. There were no differences in either SDI at baseline vs. 6m (0 ± 1 vs. 0 ± 1), or anti-dsDNA titers, C-reactive protein levels or glucocorticoid dose among any of the 3 visits.
Conclusion: In real-life clinical practice, anifrolumab improved multiple SLE activity measures at 3 and 6 months, with a substantial proportion of patients achieving LLDAS or DORIS-defined remission, along with reduced flare rates and background immunosuppressive use, and no new major safety concerns.
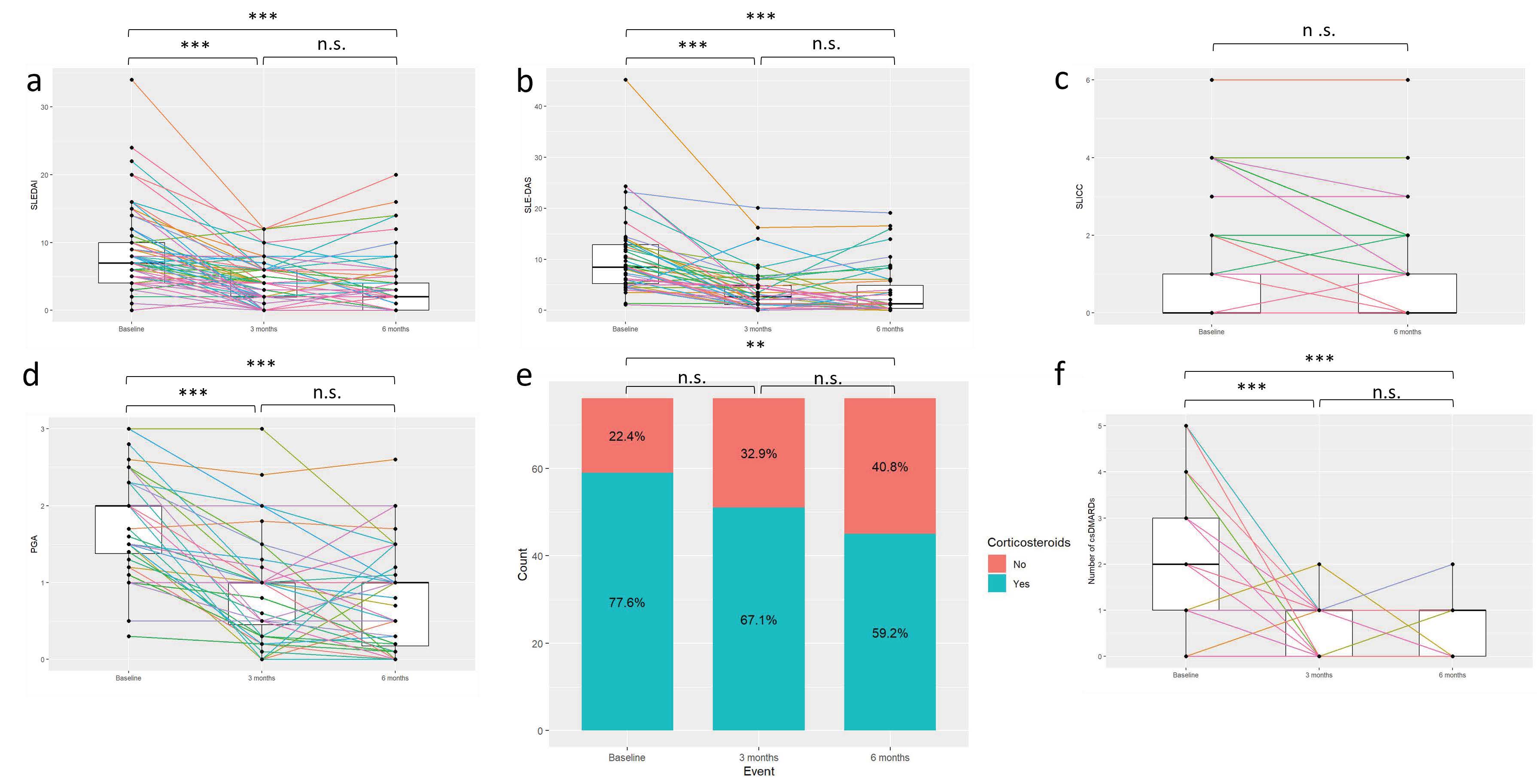 Figure 1: Statistically significant differences in disease indices between the baseline visit and the visits at 3 and 6 months after the start of anifrolumab. p-values significance level: n.s.: > 0.5; * < 0.05–0.01; ** < 0.01–0.001; *** < 0.001. a): SLEDAI – Systemic Lupus Erythematosus Disease Activity Index (n = 77); b) SLE-DAS – Systemic Lupus Erythematosus Disease Activity Score (n = 43); c) SLICC – Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (n = 73); d): PGA – Physician Global Assessment (n = 52); e) Glucocorticoids yes/no (n = 76); f) Number of disease modifying antirheumatic drugs with anifrolumab (n = 76).
Figure 1: Statistically significant differences in disease indices between the baseline visit and the visits at 3 and 6 months after the start of anifrolumab. p-values significance level: n.s.: > 0.5; * < 0.05–0.01; ** < 0.01–0.001; *** < 0.001. a): SLEDAI – Systemic Lupus Erythematosus Disease Activity Index (n = 77); b) SLE-DAS – Systemic Lupus Erythematosus Disease Activity Score (n = 43); c) SLICC – Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (n = 73); d): PGA – Physician Global Assessment (n = 52); e) Glucocorticoids yes/no (n = 76); f) Number of disease modifying antirheumatic drugs with anifrolumab (n = 76).
.jpg) Figure 2: Statistically significant differences in response indices and discontinuation between baseline and the visits at 3 and 6 months after the initiation of anifrolumab. p-values significance level: n.s.: > 0.5; * < 0.05–0.01; ** < 0.01–0.001; *** < 0.001. a) DORIS – Definition of Remission in Systemic Lupus Erythematosus (n = 66); b): LLDAS – Lupus Low Disease Activity State (n = 64); c): 50% response (n = 63); d) Serologic response (n = 63); e) Treatment discontinuation; f) Reasons for treatment discontinuation.
Figure 2: Statistically significant differences in response indices and discontinuation between baseline and the visits at 3 and 6 months after the initiation of anifrolumab. p-values significance level: n.s.: > 0.5; * < 0.05–0.01; ** < 0.01–0.001; *** < 0.001. a) DORIS – Definition of Remission in Systemic Lupus Erythematosus (n = 66); b): LLDAS – Lupus Low Disease Activity State (n = 64); c): 50% response (n = 63); d) Serologic response (n = 63); e) Treatment discontinuation; f) Reasons for treatment discontinuation.
To cite this abstract in AMA style:
Carrión-Barberà I, Salman Montes T, Triginer L, Font-Urgelles J, Riveros frutos A, Garrote Corral S, Garcia-Villanueva M, de Frías Polo C, Galindo-Izquierdo M, Magallares B, Hernández-martín A, Fragío Gil J, Sandoval Moreno S, Ramos Giraldez C, Cortés-Hernández J, Díez García E, Moriano C, Vela Casasempere P, Bernabeu Gonzalvez M, Altabás-González I, Hernández-Baldizón S, Riancho L, Ros Vilamajo I, Marras Fernández-Cid C, Piqueras García M, Garcia-Aparicio A, Garijo Bufort m, Gomez-Puerta J, Frade Sosa B, García Cirera S, Torrente-Segarra V, Sala L, Fito-Manteca C, del Val del amo N, Martínez Barrio J, Rosas Gómez de Salazar J, Heredia S, Urruticoechea-Arana a, Brandy A, Uriarte M, Pego-Reigosa J, Rúa-Figueroa I. Spanish Real-World Ambispective Multicenter Registry of Anifrolumab in Systemic Lupus Erythematosus: Efficacy and Safety at 3- and 6-Month Follow-Up (ANIFRO-Reu Study) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/spanish-real-world-ambispective-multicenter-registry-of-anifrolumab-in-systemic-lupus-erythematosus-efficacy-and-safety-at-3-and-6-month-follow-up-anifro-reu-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/spanish-real-world-ambispective-multicenter-registry-of-anifrolumab-in-systemic-lupus-erythematosus-efficacy-and-safety-at-3-and-6-month-follow-up-anifro-reu-study/
