Session Information
Date: Tuesday, October 28, 2025
Title: (2437–2469) Systemic Lupus Erythematosus – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Hydroxychloroquine (HCQ) is a cornerstone of systemic lupus erythematosus (SLE) management, but long-term use carries the risk of retinopathy. While prior studies have identified risk factors for HCQ-induced retinopathy and described varying degrees of progression even after drug discontinuation, existing literature is limited by small sample sizes. We aimed to characterize a cohort of SLE patients with confirmed HCQ retinopathy to identify factors associated with progression after discontinuation.
Methods: We queried an urban multi-ethnic lupus cohort (all ACR/SLICC or EULAR criteria longitudinally followed) for patients who had evidence of HCQ-induced retinopathy and identified 59 of 1,530 with documented retinopathy diagnoses. Of these, 28 had records available to confirm the onset of retinopathy and at least one ophthalmology follow-up to assess for progression. We conducted a chart review of these 28 patients and captured cumulative HCQ exposure, ophthalmologic screening adherence, and SLE activity preceding and following drug cessation. Ophthalmology notes were reviewed to assess for stability or progression of OCT imaging and/or Humphrey Visual Fields after discontinuation.
Results: Among 28 patients with confirmed HCQ retinopathy, 13 (46%) were White, 9 (32%) Asian, 5 (18%) Black, and 1 (4%) other; 9 patients (32%) identified as Hispanic; 26 (93%) were female. The average age at diagnosis was 53.3 years. The mean cumulative HCQ exposure across all patients with retinal toxicity was 1,827 g (SD 1,442 g) over a mean of 14.0 years (SD 8.1). Most patients (57%) were seeing ophthalmology at least yearly, while a smaller proportion (18%) had screening lapses preceding their diagnosis. At diagnosis of retinopathy, the mean SLEDAI-2K score was 2.3, and the average SLICC Damage Index was 3.13. Retinal toxicity progressed in 35% of patients despite drug discontinuation. Patients who progressed were significantly older than those who did not (60.3 vs 49.7 years, p = 0.033). No statistically significant associations were found between retinopathy progression and race, ethnicity, cumulative HCQ exposure, duration of therapy, SLICC Damage Index, SLEDAI-2K or ophthalmology screening interval.
Conclusion: In this SLE-specific cohort with confirmed HCQ-induced retinopathy, retinal toxicity progressed in a third of patients despite HCQ discontinuation. There was a significant association between age and retinopathy progression despite equivalence of cumulative dosing and ophthalmologic screening intervals. These findings support the consideration of more frequent monitoring in older patients.
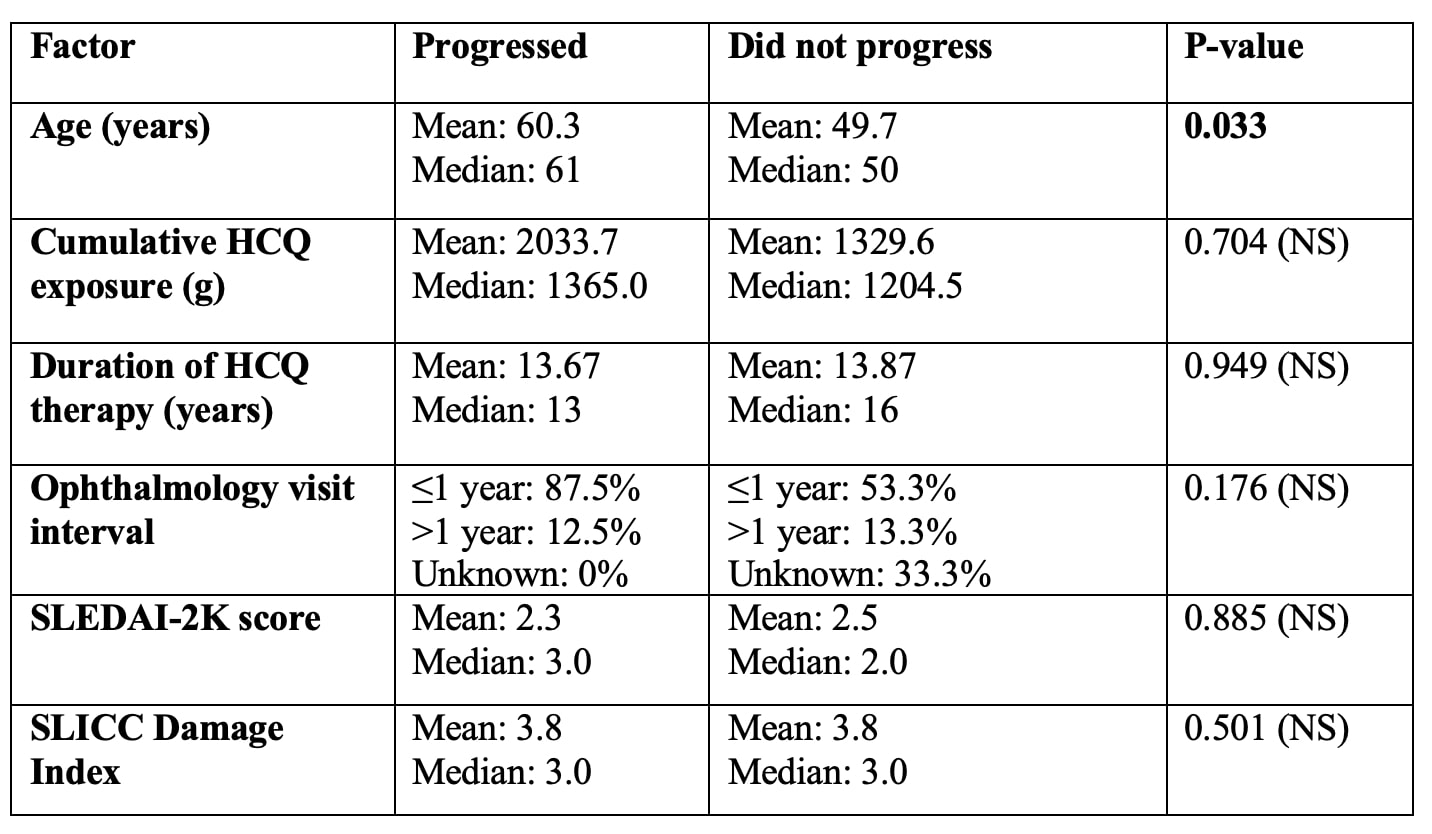 Table 1. Demographic characteristics of cohort.
Table 1. Demographic characteristics of cohort.
To cite this abstract in AMA style:
Gutowski E, Dai J, Carter E, Cohen B, Buyon J, Tseng C, Masson M, Saxena A, Belmont H, Colcombe J, Modi Y, Lee C, Izmirly P. Hydroxychloroquine-Induced Retinopathy in Systemic Lupus Erythematosus: Predictors of Progression Following Drug Discontinuation [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/hydroxychloroquine-induced-retinopathy-in-systemic-lupus-erythematosus-predictors-of-progression-following-drug-discontinuation/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hydroxychloroquine-induced-retinopathy-in-systemic-lupus-erythematosus-predictors-of-progression-following-drug-discontinuation/

.jpg)