Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Psoriatic arthritis (PsA) is a heterogeneous disease affecting joints, entheses, skin, nails, and spine. While treatment options have expanded, TNF inhibitors (TNFis) remain the most common first-line biologic in Spain due to favorable efficacy, safety, and reimbursement. However, evidence on biologic therapeutic sequencing remains limited, particularly regarding effectiveness and drug persistence.The Manhattan study (CNTO1959PSA4009) assessed persistence, effectiveness, and tolerability of guselkumab (GUS), an anti-IL-23 inhibitor, compared to a second TNFi in adults with PsA who had failed to a prior first-line TNFi therapy.
Methods: Manhattan is an ongoing ambispective, observational study across 37 Spanish centers. It includes PsA patients who switched to GUS or a second-line TNFi following first-line TNFi use. Among 193 recruited, 175 (98 on GUS and 77 on TNFi) patients were evaluated in this interim analysis regarding effectiveness and tolerability. Baseline data, and prior/concomitant treatments were recorded. Endpoints included rates of disease activity (minimal (MDA), DAPSA low (LDA)/remission), presence of dactylitis, nail psoriasis, enthesitis measurement (by the Leeds Enthesitis Index [LEI]), functioning and disability using the Health Assessment Questionnaire (HAQ), mean changes in psoriatic body surface area (BSA), and tender (TJC), and swollen joint count (SJC). Adverse events (AE) and drug-related events were recorded. Effectiveness and tolerability interim results up to week (W) 52 are presented.
Results: Baseline characteristics were similar across groups (Table 1). MDA achievements increased to 52.3% (GUS) and 33.3% (TNFi) at W52 (Fig. 1A). DAPSA Remission/LDA rates rose from 18.8% at baseline to 66.7% at W52 (GUS), and from 23.5% to 62.2% (TNFi), respectively (Fig. 1B). TJC improved in both groups, showing mean absolute changes from baseline of –2.4 (W12) and -3.1 (W52) for GUS, and of –1.5 (W12) to –3.5 (W52) for TNFi. Similarly, SJC decreased over time: mean absolute changes of –1.9 and –2.1 (GUS), and of –1.2 and –2.4 (TNFi), at W12 and W52, respectively. Dactylitis prevalence decreased in both groups (Fig. 2A) and it disappeared in 46.2% (6/13) patients W12, 80.0% (8/10) W24, and 71.4% (5/7) W52 (GUS), and in 88.9% (8/9) W12, 75.0% (6/8) W24, and 100.0% (5/5) at W52 (TNFi). LEI scores marginally improved with both treatments, whereas HAQ remained stable with GUS and slightly improved with TNFi (from 1.0 to 0.8 [W52]) (Fig. 2B and C).Nail psoriasis mean absolute changed from baseline: –1.8 (W12), –3.2 (W24), and –10.5 (W52) in TNFi group, versus –0.9, –1.8, and –1.3, respectively, in the GUS group. BSA improvement favored GUS, showing mean absolute changes of –2.6 (W12), –3.2 (W24), and –3.9 (W52), versus –0.8, –1.1, and –1.7, respectively in the TNFi group. Reported AEs in 35 patients: 19 (19.4%) GUS and 16 (20.8%) TNFi. Of total AEs, 4 (14.8%) and 7 (31.8%) were considered drug-related, respectively.
Conclusion: Guselkumab and second-line TNFis showed effectiveness through 52 weeks in diverse domains. GUS provided earlier MDA response and greater improvement in both skin outcomes and LDA/remission, supporting its use as a durable second-line option
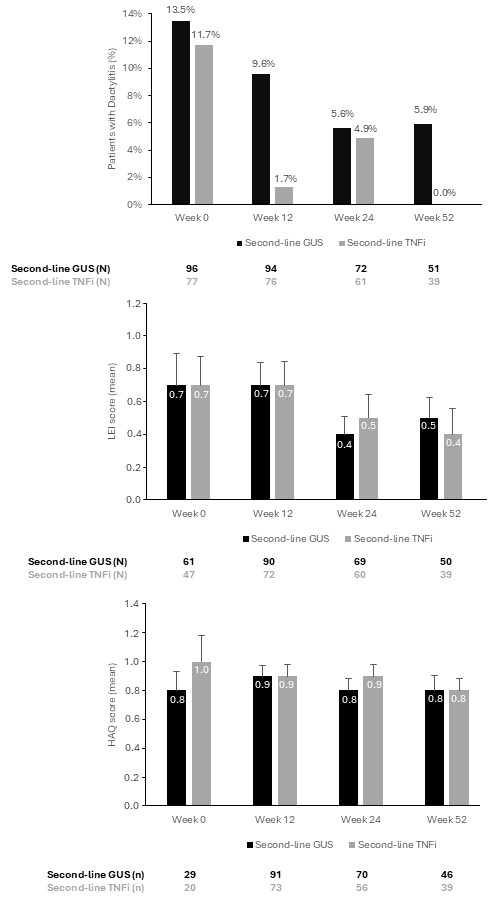 Table 1. Demographic characteristics.
Table 1. Demographic characteristics.
.jpg) Figure 1. a) Percentage of patients achieving minimal disease activity (MDA) in second-line treatment (GUS or TNFi at week 0, week 12, 24 and 52). MDA was achieved if 5 of 7 criteria were met: TJC ≤1; SJC ≤1; BSA ≤ 3%; patient’s assessment of pain by visual analogue scale (VAS) ≤15 mm; patient’s global assessment of disease activity by VAS ≤20 mm; Health Assessment Questionnaire ≤0.5; and tender entheseal points ≤ 1 (assessed by the Leeds Enthesitis Index). b) percentage of patients achieving remission and low disease activity, moderate or high disease activity.
Figure 1. a) Percentage of patients achieving minimal disease activity (MDA) in second-line treatment (GUS or TNFi at week 0, week 12, 24 and 52). MDA was achieved if 5 of 7 criteria were met: TJC ≤1; SJC ≤1; BSA ≤ 3%; patient’s assessment of pain by visual analogue scale (VAS) ≤15 mm; patient’s global assessment of disease activity by VAS ≤20 mm; Health Assessment Questionnaire ≤0.5; and tender entheseal points ≤ 1 (assessed by the Leeds Enthesitis Index). b) percentage of patients achieving remission and low disease activity, moderate or high disease activity.
.jpg) Figure 2. a) Percentage of patients with dactylitis, b) representation of the mean LEI score, c) representation of HAQ score in second-line treatment (GUS or TNFi at week 0, week 12, 24 and 52).
Figure 2. a) Percentage of patients with dactylitis, b) representation of the mean LEI score, c) representation of HAQ score in second-line treatment (GUS or TNFi at week 0, week 12, 24 and 52).
To cite this abstract in AMA style:
Gonzalez Molina R, JOVEN B, Llanes M, Hernandez V, Moreno M, Blanco E, Marin C, Mateo L, Salles Lizarzaburu M, Steiner M, Nuño L, Fernández-Nebro A, Veroz González r, Urruticoechea-Arana a, Cervantes E, Giner e, Fernandez de la fuente Burson L, Erra A, Camacho O, Garcia E, Hernandez A, Malave J, Orpinell L, Ros Vilamajo I, Castro S, Lopez Viejo P, Sarabia L, García Portales R, Cabezon J, Moreno Gil P, Maceiras F, Pielfort D, Diaz-Castroverde S, Cuervas-mons M, Pinto-Tasende J, Muñoz S, Ramirez J. Effectiveness and Tolerability of Guselkumab or TNF inhibitors as Second-Line Treatment After Receiving a TNF inhibitor as First-Line Therapy to Treat Active Psoriatic Arthritis: 52-Week Interim Data from the Manhattan Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/effectiveness-and-tolerability-of-guselkumab-or-tnf-inhibitors-as-second-line-treatment-after-receiving-a-tnf-inhibitor-as-first-line-therapy-to-treat-active-psoriatic-arthritis-52-week-interim-data/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-and-tolerability-of-guselkumab-or-tnf-inhibitors-as-second-line-treatment-after-receiving-a-tnf-inhibitor-as-first-line-therapy-to-treat-active-psoriatic-arthritis-52-week-interim-data/
