Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Uveitis is a common extra-musculoskeletal manifestation in patients (pts) with spondyloarthritis (SpA); incidence varies with SpA type and disease duration.1–3 IL-17 has been implicated in uveitis pathogenesis.4 However, previous data demonstrating IL‑17A inhibition efficacy in managing uveitis are limited and conflicting.5–7The exposure-adjusted incidence rate (EAIR) per 100 pt-years (PY) of uveitis was lower in pts with non-radiographic and radiographic axial SpA (axSpA) randomized to bimekizumab (BKZ; 1.8/100 PY), which selectively inhibits IL-17F in addition to IL-17A, vs placebo (15.4/100 PY) after 16 weeks in phase (Ph)3 studies.8 Previously reported long-term data over ~2 years (data cut-off: Jul 2023) showed that uveitis rates remained low in BKZ-treated pts with axSpA (1.3/100 PY) and psoriatic arthritis (PsA; 0.1/100 PY).9We report updated uveitis incidence in BKZ-treated pts with axSpA or PsA in Ph2b/3 studies with an additional year of treatment duration in the Ph3 studies (data cut-off in axSpA: Sep 2024; PsA: Aug 2024).
Methods: Data are reported for two pools, each comprising one Ph2b and two Ph3 studies and their open-label extensions, in pts with axSpA and PsA, respectively (Figure 1).Uveitis events were identified using the preferred terms “autoimmune uveitis”, “iridocyclitis”, “iritis”, and “uveitis”, classified using the MedDRA v19.0; “acute anterior uveitis” was not a specific preferred term available in MedDRA v19.0. Uveitis rates and EAIR/100 PY for pts who received ≥1 BKZ 160 mg every four weeks (Q4W) dose are reported.
Results: Pts with axSpA (Nf848) and PsA (Nf1,409) had a mean (SD) age of 40.3 (11.9) and 49.3 (12.4) years, respectively. Mean (SD) time since diagnosis was 6.1 (7.8) years in pts with axSpA and 7.0 (8.0) in pts with PsA. Of pts with axSpA, 130 (15.3%) had a history of uveitis (PsA: 21 [1.5%]). Most pts with axSpA were HLA-B27 positive (717/848 [84.6%]).In pts with axSpA across the pooled Ph2b/3 data, BKZ exposure was 2,748.9 PY. In total, uveitis occurred in 33/848 (3.9%; EAIR [95% CI]: 1.2/100 PY [0.8, 1.7]) pts overall and in 20/130 (15.4%; 5.0/100 PY [3.0, 7.7]) pts with history of uveitis. In pts without a history of uveitis, 13/718 (1.8%; 0.6/100 PY [0.3, 1.0]) pts had uveitis events (Figure 2). Most events were mild/moderate, one was severe; two (0.2%) patients discontinued treatment due to uveitis.Incidence of uveitis in pts with PsA was low across the pooled Ph2b/3 data (total BKZ exposure: 4,264.7 PY); uveitis occurred in 4/1,409 (0.3%; 0.1/100 PY [0.0, 0.2]) pts overall; two had a history of uveitis. No uveitis events led to treatment discontinuation.
Conclusion: With an additional year of BKZ treatment from previously reported data, the incidence of uveitis in pts with SpA receiving BKZ long-term, over 3 years, remained low.References: 1. Robinson PC. Arthritis Rheumatol 2015;67:140–51; 2. López-Medina C. RMD Open 2019;5:e001108; 3. De Vicente Delmás A. RMD Open 2023;9:e002781; 4. Hysa El. Eur. J. Clin. Invest 2021;51:e13572; 5. Dick AD. J. Ophthalmol 2013;120:777–87; 6. Kwon OC. Rheumatol 2025;64:588–96; 7. Letko E. J. Ophthalmol 2015;122:939–48; 8. Brown MA. Ann Rheum Dis 2024;1722–30; 9. van der Horst‑Bruinsma IE. Arthritis Rheumatol 2024;76(suppl 9). Abstract 2351.
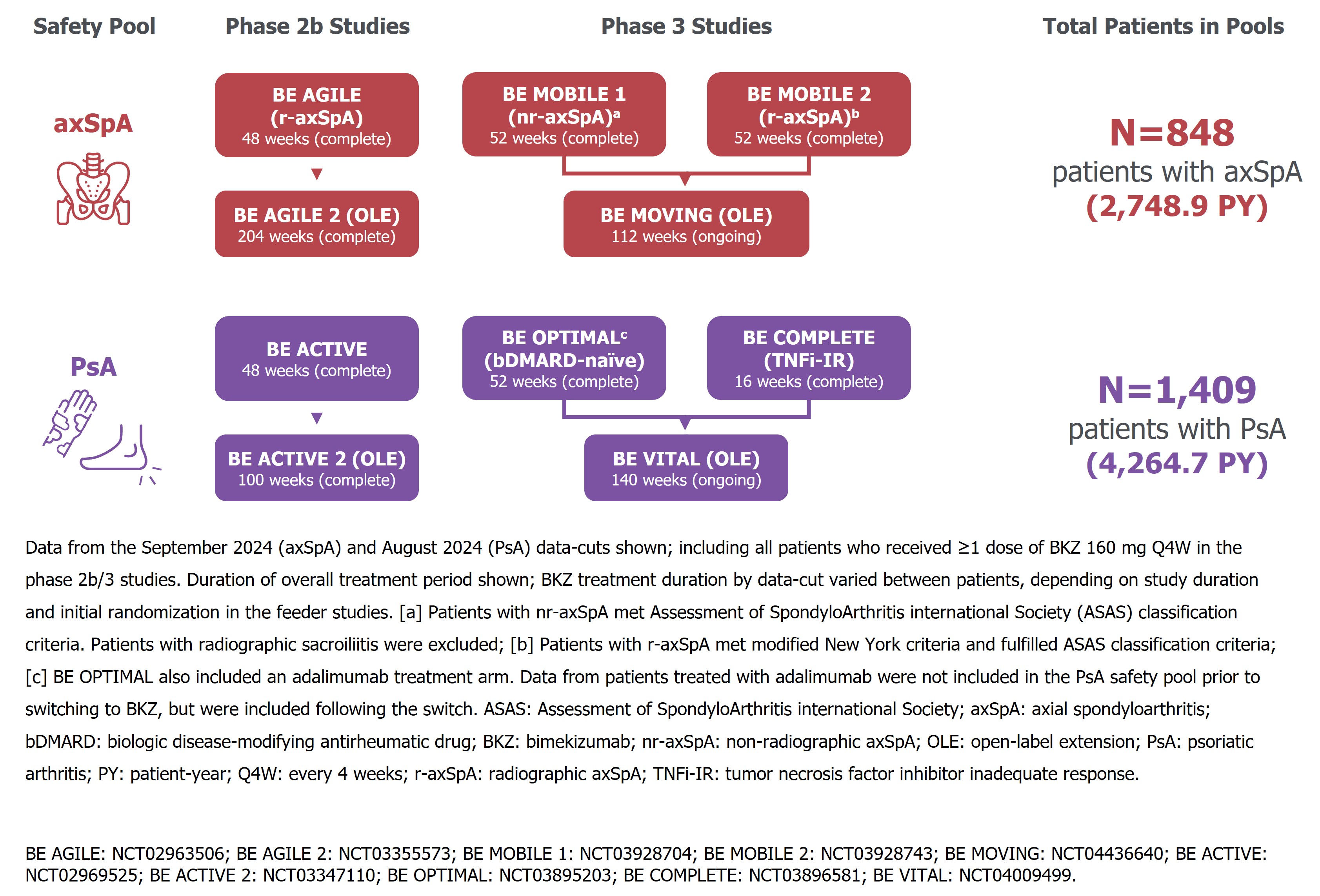 Figure 1. Two safety pools (axSpA, PsA) of patients treated with BKZ 160 mg Q4W across six phase 2b/3 studies and their open-label extensions
Figure 1. Two safety pools (axSpA, PsA) of patients treated with BKZ 160 mg Q4W across six phase 2b/3 studies and their open-label extensions
.jpg) Figure 2. Incidence of uveitis in patients with axSpA stratified by history of uveitis
Figure 2. Incidence of uveitis in patients with axSpA stratified by history of uveitis
To cite this abstract in AMA style:
Rudwaleit M, van der Horst-Bruinsma I, van Gaalen F, Haroon N, Gensler L, Manente M, Prajapati C, White K, Deodhar A, Brown M. Long-Term Uveitis Rates with Bimekizumab Treatment Across Pooled Phase 2b and Phase 3 Studies in Patients with Axial Spondyloarthritis or Psoriatic Arthritis: 3-Year Update [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/long-term-uveitis-rates-with-bimekizumab-treatment-across-pooled-phase-2b-and-phase-3-studies-in-patients-with-axial-spondyloarthritis-or-psoriatic-arthritis-3-year-update/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-uveitis-rates-with-bimekizumab-treatment-across-pooled-phase-2b-and-phase-3-studies-in-patients-with-axial-spondyloarthritis-or-psoriatic-arthritis-3-year-update/
