Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Interleukin-17 (IL-17) inhibitors, such as secukinumab and ixekizumab, are used for their anti-inflammatory effects in conditions like psoriasis and ankylosing spondylitis. IL-17 signaling has been implicated in cardiovascular disease pathogenesis, including atrial fibrillation and other arrhythmias. This study evaluates the incidence and characteristics of arrhythmias associated with IL-17 inhibitors using the FDA Adverse Event Reporting System (FAERS).
Methods: The FAERS database was searched for cardiac arrhythmias (Tachycardia, bradycardia, atrial fibrillation, QRS complex abnormalities, QT interval abnormalities, bundle branch block (BBB), atrioventricular blocks (AV blocks), and supraventricular tachycardia (SVT)) associated with the use of IL-17 inhibitors (secukinumab, ixekizumab, bimekizumab). Disproportionality analysis was done and data were reported as frequencies and as reporting odds ratios (ROR) with a 95% confidence interval (95% CI). RORs were only reported for adverse reactions with frequencies of 3 or more.
Results: We analyzed 30,179,725 adverse event reports from the FAERS database. A total of 181,052 reports were associated with the use of the three anti-IL-17 drugs; 60.9% of them were females, and 33.2% were in the age group between 18-64 years. Cardiac arrhythmias were reported in 0.54% of cases. Most of the cases were associated with secukinumab use (88.9%), followed by ixekizumab (9.5%). Among bimekizumab users, atrial fibrillation was the most common arrhythmia and accounted for 57.1% of all arrhythmias included in our analysis (Figure 1). However, when tested using ROR, the reporting odds of atrial fibrillation weren’t higher in bimekizumab users compared to other drugs users. On the other hand, the most reported arrhythmias among secukinumab were tachycardia (45.6%) and bradycardia (41.9%) (Figure 2). When compared to other anti IL-17 medications, secukinumab was significantly associated with higher odds of multiple arrhythmias, including tachycardia (ROR=12.27; 95% CI: 8.623-17.47), bradycardia (ROR=12.27; 95% CI: 4.469 – 34.91), atrial fibrillation (ROR=7.101; 95% CI: 5.317 – 9.484), Supraventricular Tachycardia (ROR=3.747; 95% CI: 1.208 – 11.62) and AV block (ROR=8.118; 95% CI: 2.833 – 23.26) (Table 1).
Conclusion: This study suggests that IL-17 inhibitors, particularly secukinumab, may be associated with increased reporting of cardiac arrhythmias, including tachycardia, bradycardia, atrial fibrillation, supraventricular tachycardia, and atrioventricular block. These findings support the need for further investigation into the cardiovascular safety of IL-17 inhibitors to guide clinical decision-making.
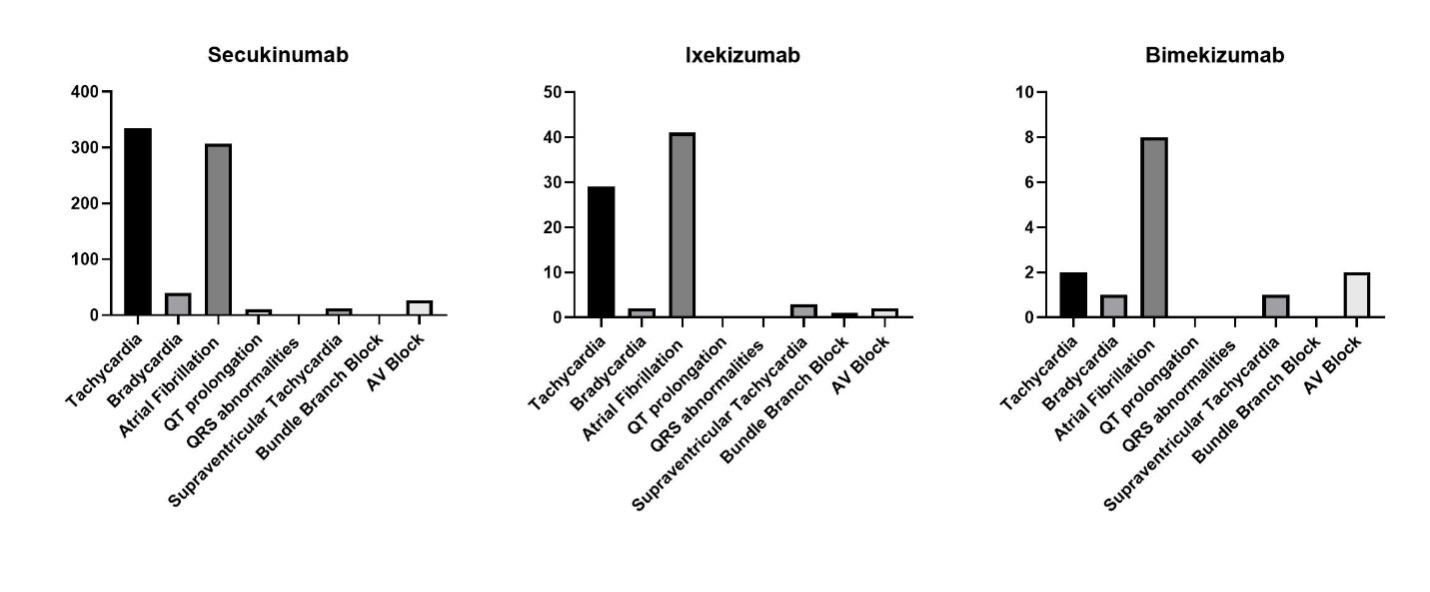 Figure 1. Frequency of arrhythmias
Figure 1. Frequency of arrhythmias
.jpg) Figure 2. Reported Odds Ratio (ROR) of Anti-IL 17 drugs
Figure 2. Reported Odds Ratio (ROR) of Anti-IL 17 drugs
.jpg) Table 1. ROR of anti-IL 17 drugs
Table 1. ROR of anti-IL 17 drugs
To cite this abstract in AMA style:
Khanfar A, Hamdan O, Regmi A, Goreja M, Ocon A. Risk of Arrhythmias Following IL-17 Inhibitor Use; A Pharmacosurvellience Study of FDA Adverse Event Reporting System (FAERS) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/risk-of-arrhythmias-following-il-17-inhibitor-use-a-pharmacosurvellience-study-of-fda-adverse-event-reporting-system-faers/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-arrhythmias-following-il-17-inhibitor-use-a-pharmacosurvellience-study-of-fda-adverse-event-reporting-system-faers/
