Session Information
Date: Tuesday, October 28, 2025
Title: (2106–2123) Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science Poster II
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Treatment with aromatase inhibitors (AIs) in patients with breast cancer is associated with accelerated bone mineral density (BMD) loss, leading to an increased risk of fracture, even in patients with BMD levels in the osteopenic range. In this context, treatment algorithms with higher BMD thresholds (T-score ≤ -2.0) than general population recommendations have been proposed. However, the implementation of these recommendations in clinical practice has been poorly evaluated.
Methods: Prospective observational study. Postmenopausal women with breast cancer receiving AIs who initiated antiresorptive therapy between October 2021 and April 2025 were included. Demographic, laboratory, and bone densitometry data (baseline and at 18 months) were collected from electronic medical records. Two groups were established based on T-score at initiation of antiresorptive therapy: T-score ≤ -2.0 and > -2.5, and T-score ≤ -2.5. Comparisons between groups were performed using the Mann-Whitney test (for two variables) or the Kruskal-Wallis test (for three or more variables).
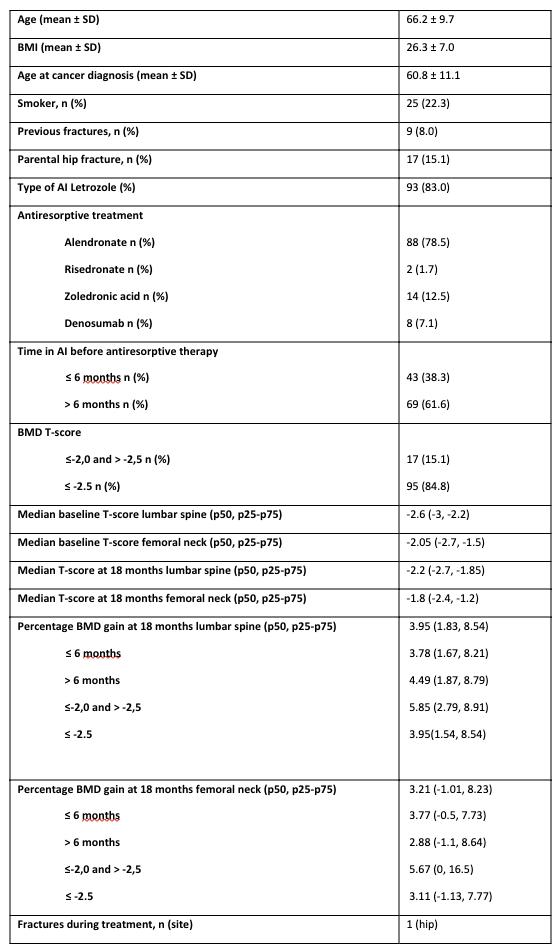
Results: A total of 220 women met inclusion criteria by April 2025; 129 completed the 18-month follow-up, with 17 excluded due to discontinuation of antiresorptive therapy before 18 months. Mean age was 66.2 ± 9.7 years; 88 (78.5%) were treated with alendronate. 38.3% had been on AIs for less than 6 months before initiating antiresorptive therapy. 84.8% started therapy with a T-score ≤ -2.5 and 15.1% with a T-score ≤ -2.0 and > -2.5. At 18 months, the median percentage increase in BMD at the lumbar spine (LS) was 3.9% (1.8; 8.5), and at the femoral neck (FN) 3.2% (-1.0; 8.2). BMD gain was higher in the group initiating antiresorptive treatment at T-score ≤ -2.0 and > -2.5 compared to the ≤ -2.5 group, both at LS and FN (5.85% vs. 3.95%, p=0.31 at LS; and 5.67% vs. 3.11%, p=0.12 at FN). No statistically significant differences in BMD gain were found based on AI exposure duration (< or > 6 months) prior to antiresorptive therapy. Similarly, no significant differences were observed based on osteoporosis risk factors or the type of antiresorptive agent used.
Conclusion: Our findings suggest a greater increase in BMD among patients who begin antiresorptive therapy at a T-score of ≤ -2.0 compared to those who start at the general population threshold of ≤ -2.5. Initiating antiresorptive therapy in AI-treated patients is recommended regardless of the duration of AI use, as it does not appear to significantly affect BMD gain.
To cite this abstract in AMA style:
Paz Liñeira L, Villapún Burgoa B, Castrejón Fernández I, álvaro-Gracia álvaro J, González Hernández T, López Tarruella S, Jerez gilarranz Y. Prevention of Bone Mass Loss Associated With Aromatase Inhibitors in Breast Cancer. A Study in Real-World Clinical Practice. [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/prevention-of-bone-mass-loss-associated-with-aromatase-inhibitors-in-breast-cancer-a-study-in-real-world-clinical-practice/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prevention-of-bone-mass-loss-associated-with-aromatase-inhibitors-in-breast-cancer-a-study-in-real-world-clinical-practice/