Session Information
Date: Tuesday, October 28, 2025
Title: (2106–2123) Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science Poster II
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Osteonecrosis of the jaw (ONJ) is defined as avascular necrosis of bone located in the maxillofacial region with the presence of exposed bone or bone accessible through an extra or intraoral fistula that does not heal within 8 weeks and excludes osteoradionecrosis secondary to radiotherapy. ONJ can be secondary to drugs and it is referred to Medication-related osteonecrosis of the jaw (MRONJ). To define a MRONJ is required a current or previous treatment with antiresorptive therapy alone or in combination with immune modulators or antiangiogenic medications.With this study we want to analyse which drugs are associated with the development of MRONJ as well as to describe the clinical and epidemiological characteristics of patients diagnosed with MRONJ.
Methods: A descriptive, observational and retrospective study was carried out at the Santa Lucia hospital in Cartagena. Individuals with MRONJ was collected from 2015 to 2024 at the Santa Lucia Hospital, in Cartagena. Clinical-epidemiological variables were collected and a statistical analysis was carried out using SPSSv21.
Results: 24 individuals with a diagnosis of MRONJ was collected. Fifty-six per cent of the blind were women and the remainder (44 per cent) were men. The average age was 69 +- 13.2 years. Of these patients 56% were hypertensive, 20% diabetic, 32% had dyslipidaemia and 12% had stage 3-4 chronic kidney disease. 78.1% of the individuals had an underlying oncological disease and 4.9% had a rheumatological disease. Of all people, 22% had previously had an invasive dental procedure. At the time of MRONJ diagnosis, 100% of the individuals were being treated with antiresorptive drugs: 85.2% were treated with bisphosphonates and 14.8% with denosumab. The average treatment time with these drugs was 4.15 +- 3.7 years. Of those on bisphosphonate therapy, 66.6% were on intravenous bisphosphonates (zoledronic acid) and the remainder on oral therapy (11,1% risendronate, 11,1% Alendronate and 11,1% other). These treatments were indicated as oncological treatment in 69.23% of cases, while only 26.9% were used as anti-osteoporotic treatment. In 3.87% of cases they were indicated as a treatment for benign bone disease. In the oncology indication group, 84.6% of the MRONJ cases were due to the use of zoledronate, while in the anti-osteoporotic indication group only 14.2% of the MRONJ cases were caused by zoledronate. These differences were statistically significant (p< 0.001). Therefore, the development of ONMR in patients treated with zoledronate was more frequent when it was used as an oncology treatment. In the anti-osteoporotic indication group the 57,8% of the patients with MRONJ were on oral bisphosphonate therapy and 28,57% with denosumab.
Conclusion: The drug most associated with the development of MRONJ in our sample of patients was zoledronic acid, being the development of this entity much more frequent when it is administered as an oncological treatment. Therefore, it could be hypothesised that the risk of MRONJ does not depend so much on the drug as on the indication and administration schedule, but more studies are needed to clarify the association between MRONJ and the different drugs.
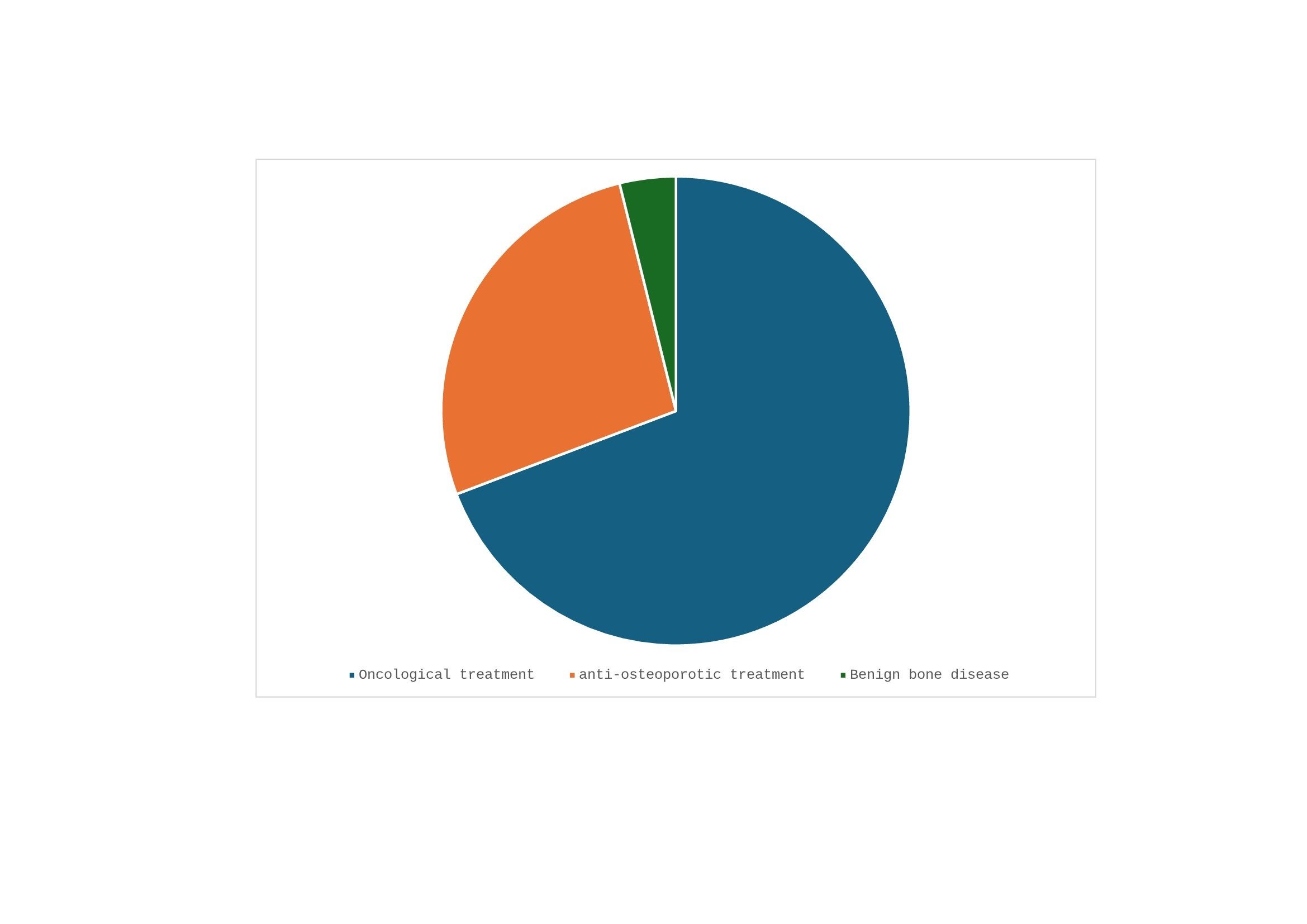 1. Distribution of cases of MRONJ according to antiresorptive drug and clinical indication.
1. Distribution of cases of MRONJ according to antiresorptive drug and clinical indication.
.jpg) 2. Distribution of patients (with MRONJ ) treated with bisphosphonates according to clinical indication.
2. Distribution of patients (with MRONJ ) treated with bisphosphonates according to clinical indication.
To cite this abstract in AMA style:
Monleon Acosta E, Pérez González A, Rodríguez Fernández J, Manuel Hernández P, Oliva Ruiz M, Andreu Ubero J, Castillo Dayer P, Albaladejo Paredes G, Fernández Díaz C, Egea Fuentes A, Fernández Salamanca M, Cogolludo Campillo V. Which drugs are associated with the development of osteonecrosis of the jaw? clinical and epidemiological analysis of a cohort of patients diagnosed with osteonecrosis of the jaw. [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/which-drugs-are-associated-with-the-development-of-osteonecrosis-of-the-jaw-clinical-and-epidemiological-analysis-of-a-cohort-of-patients-diagnosed-with-osteonecrosis-of-the-jaw/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/which-drugs-are-associated-with-the-development-of-osteonecrosis-of-the-jaw-clinical-and-epidemiological-analysis-of-a-cohort-of-patients-diagnosed-with-osteonecrosis-of-the-jaw/
