Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Primary results from the phase 4 MOSAIC trial demonstrated that apremilast (APR) improved inflammation in patients (pts) with PsA based on PsA MRI scoring of the hand. Pts also underwent optional dynamic contrast-enhanced MRI (DCE-MRI), which evaluated perfusion within soft tissues and bone marrow of the hand to estimate the degree of joint and soft tissue inflammation in dactylitis, a hallmark of PsA characterized by swelling and inflammation of digits. We report the efficacy of APR in pts from the MOSAIC trial with pre-existing dactylitis.
Methods: MOSAIC (NCT03783026) was a multicenter, single-arm, open-label study that evaluated pts with PsA (duration: 3 mo to 5 y; met CASPAR criteria) who received oral APR 30 mg twice daily for up to 48 weeks. Conventional MRIs of pts with clinically reported dactylitis at baseline (BL; digit not specified) were read by an independent radiologist to determine the most affected dactylitic finger. Another 2 independent readers, blinded to time point and clinical information, reviewed DCE-MRI using the Dynamic Contrast-Enhanced MRI Quantification (DEMRIQ) method; the volume of contrast-enhanced voxels in dactylitic fingers and joints were calculated: volume of enhancement (DEMRIQ-Vol), volume of enhancement × mean maximum enhancement (DEMRIQ-ME), and volume of enhancement × mean initial rate of enhancement (DEMRIQ-IRE) within each region of interest around each digit and joint. We report changes in these 3 DCE-MRI outcomes in the MRI-determined most-affected joint of the dactylitic finger, as well as in clinical disease activity measured by the Leeds Dactylitis Index (LDI) and the PsA Disease Activity Score (PASDAS) at Week (W) 24 and W48 (clinical assessments not blinded to time point).
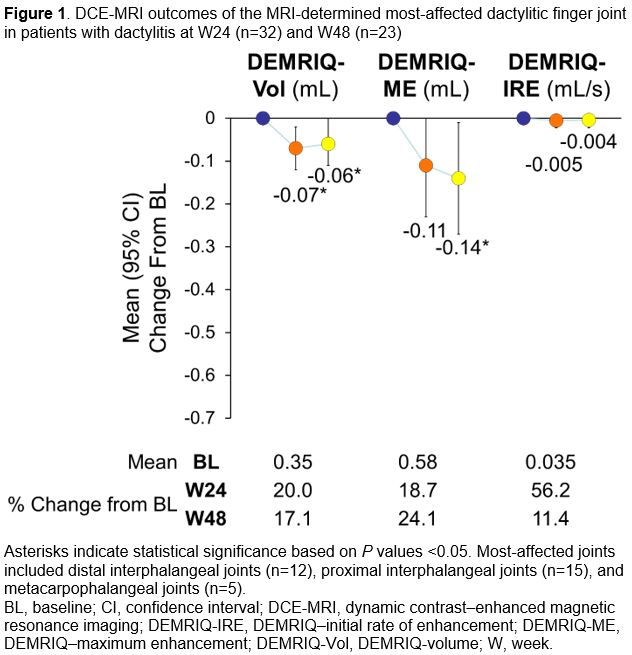
Results: Thirty-two pts in MOSAIC had both BL dactylitis and W24 imaging (mean age: 41.6 y; mean PsA duration: 1.9 y; moderate Clinical Disease Activity Index for PsA [cDAPSA] score [ >13 to ≤27]: 46.9%; high cDAPSA score [ >27]: 50.0%); 23 had W48 imaging. Statistically significant decreases in DEMRIQ-Vol (W24 and W48) and DEMRIQ-ME (W48) were observed for the MRI-determined most-affected joint (P< 0.05; Figures 1, 2). DEMRIQ-IRE showed no significant change. Statistically significant decreases in the mean (95% CI) change from BL were observed for clinical outcomes at W24 (LDI: -36.8 [-44.4, -29.2]; PASDAS: -2.3 [-2.8, -1.8]) and W48 (LDI: -39.5 [-47.8, -31.1]; PASDAS: -2.3 [-2.9, -1.8]; Figure 3). At W48, 26/28 pts (92.9%) had an LDI of 0, and 13/31 pts (41.9%) had PASDAS < 3.2 (indicating minimal disease activity). Subgroup analyses by BL disease activity will be presented.
Conclusion: There were significant reductions in DEMRIQ-ME and DEMRIQ-Vol with APR at W24 and/or W48; DEMRIQ-IRE did not show significant changes. Clinical outcomes significantly improved. DCE-MRI outcomes suggest increased capillary permeability, synovial and joint capsule volume, engorged microvasculature, and delayed contrast washout within dactylitic finger joints in PsA that improve at different rates after APR treatment and highlight the potential of using different DCE-MRI parameters to understand, quantify, and monitor the complex inflammatory load and response to treatment in pts with PsA and dactylitis.
To cite this abstract in AMA style:
Boesen m, Mease P, Maksymowych W, Lambert R, Kubassova O, Carrino J, Moradi K, Bubb M, Valenzuela G, de Vlam K, Reddy J, Chaudhari S, Zhou Z, Ostergaard M. Efficacy of Apremilast in Patients With Psoriatic Arthritis and Pre-existing Dactylitis: Dynamic Contrast-Enhanced MRI Results From the Phase 4 MOSAIC Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-apremilast-in-patients-with-psoriatic-arthritis-and-pre-existing-dactylitis-dynamic-contrast-enhanced-mri-results-from-the-phase-4-mosaic-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-apremilast-in-patients-with-psoriatic-arthritis-and-pre-existing-dactylitis-dynamic-contrast-enhanced-mri-results-from-the-phase-4-mosaic-study/

.jpg)
.jpg)