Session Information
Session Type: Abstract Session
Session Time: 1:45PM-2:00PM
Background/Purpose: MAS is a life-threatening complication of Still’s disease characterized by systemic IFNg-driven hyperinflammation. Patients with Still’s disease may present with MAS at any disease stage, including at the time of initial diagnosis. MAS episodes may also recur. Emapalumab, an anti-IFNg antibody, achieved sustained control of MAS and facilitated rapid tapering of glucocorticoids (GCs) in a phase 2 pilot study (NCT03311854). This analysis presents data by MAS presentation from an expanded population of patients treated with emapalumab for MAS in Still’s disease.
Methods: Data were pooled from 2 open-label, single-arm interventional studies (NI-0501-06 [NCT03311854] and NI-0501-14 [EMERALD; NCT05001737]) in patients with MAS in Still’s disease who had an inadequate response to high-dose GCs or investigator-assessed rapid worsening of clinical condition and/or laboratory parameters. All patients were administered a loading dose of emapalumab 6 mg/kg via intravenous infusion on Day 1; emapalumab 3 mg/kg was then administered every 3 days from Days 4–16 and twice weekly from Days 17–28 (or longer for insufficient clinical response). The primary efficacy endpoint was complete response (CR) at Week 8 (MAS clinical activity score measured on a 10 cm visual analog scale [VAS] ≤1/10 cm) and normalization of 7 MAS-related laboratory parameters. Partial response (PR) was defined as VAS < 4 cm and normalization of ≥3 abnormal baseline laboratory parameters included in the composite primary endpoint. MAS was classified as classic (first MAS diagnosis secondary to an earlier Still’s diagnosis), diagnosed at onset of Still’s disease or recurrent. MAS subgroup data was collected prospectively in EMERALD and retrospectively using patient narratives for NCT03311854.
Results: 39 patients were enrolled (31 [79.5%] females), with a median age of 12 years (range, 9 months–64 years). 84.6% of patients received concomitant biologics alone or in combination, mainly anti-interleukin-1 (66.6%) and cyclosporine A (43.6%). Twenty patients (51.3%) had MAS at Still’s disease onset, 5 (12.8%) patients had classical MAS, and 14 (35.9%) had recurrent MAS. A higher proportion of patients in EMERALD had recurrent MAS compared with NI-0501-06 (10/25 [40.0%] vs 4/14 [28.7%]) and a lesser proportion had classical MAS (2/25 [8.0%] vs 3/14 [21.4%]). CR (54.5–60.0%) and VAS ≤1 cm (76.9–100%) rates were similar across groups (Figures 1 and 2). While the recurrent group had the highest rates of non-responders at Week 8, overall response (OR) rates were still high with 8/11 patients (72.7%) achieving an OR at Week 8 and 10/13 (76.9%) achieving VAS ≤1 cm at Week 8. Changes in GC dosing from baseline to Week 8 were similar across MAS subgroups (Figure 3). Emapalumab was well tolerated. One patient with MAS at diagnosis of Still’s disease and one patient with recurrent MAS died during the study.
Conclusion: Emapalumab treatment was associated with consistent CR rates of approximately 55–60% among patients with different MAS presentations, who had an inadequate response to high-dose GCs, including patients with recurrent disease. Patients achieving VAS ≤1 cm, changes in GC dosing and safety were also similar across MAS subgroups.
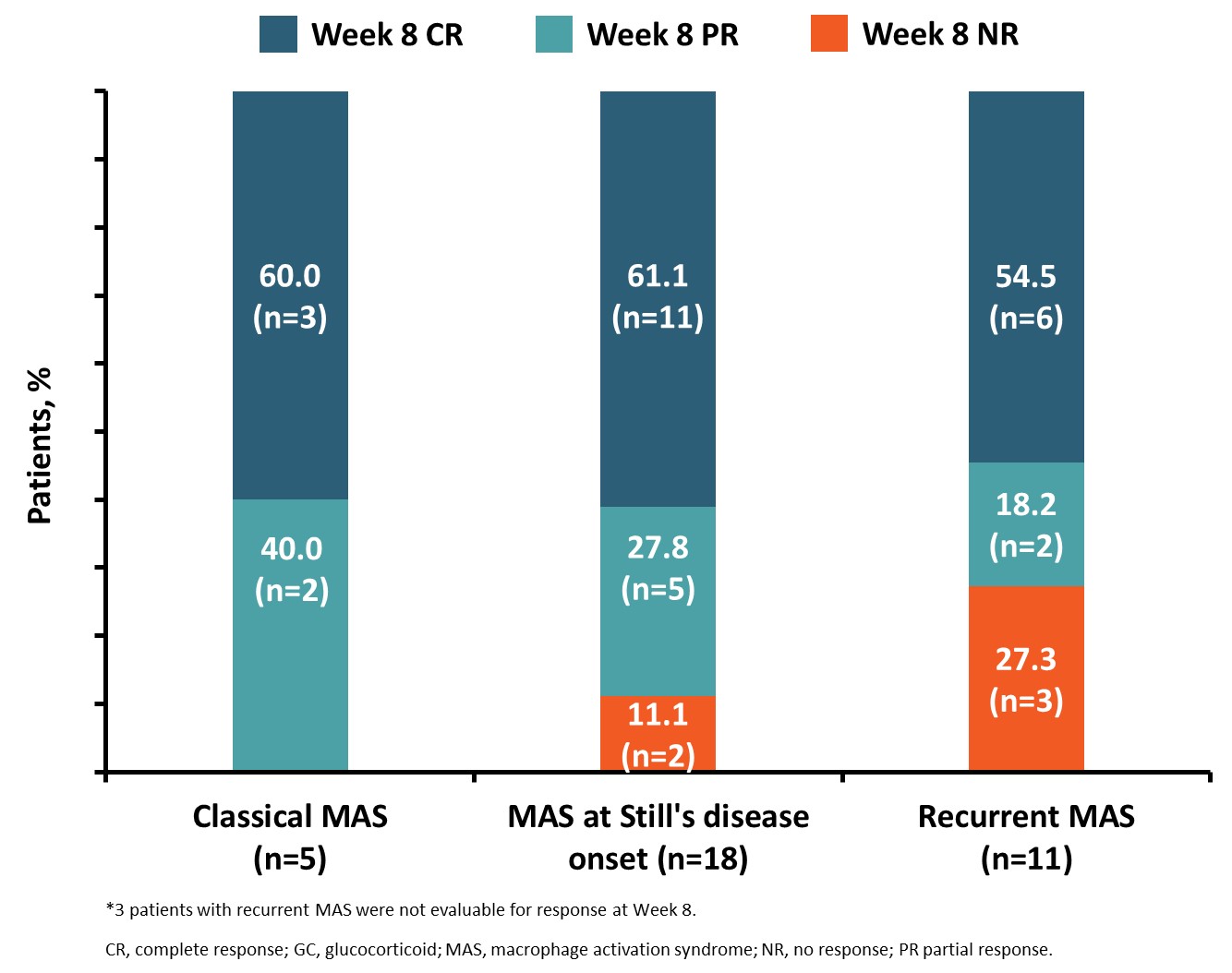 Figure 1. Week 8 responder status* by presentation subgroup amongst patients with MAS in Still’s disease with an inadequate response to high-dose GCs
Figure 1. Week 8 responder status* by presentation subgroup amongst patients with MAS in Still’s disease with an inadequate response to high-dose GCs
.jpg) Figure 2. Prevalence of VAS ≤1 cm† at Week 8 by presentation subgroup amongst patients with MAS in Still’s disease with an inadequate response to high-dose GCs
Figure 2. Prevalence of VAS ≤1 cm† at Week 8 by presentation subgroup amongst patients with MAS in Still’s disease with an inadequate response to high-dose GCs
.jpg) Figure 3. Change in GC dosing over time in patients treated with emapalumab from different MAS subgroups who had an inadequate response to high-dose GCs
Figure 3. Change in GC dosing over time in patients treated with emapalumab from different MAS subgroups who had an inadequate response to high-dose GCs
To cite this abstract in AMA style:
GROM A, Vastert S, anton J, Quartier P, Fautrel B, Brogan P, Behrens E, Elder M, Minoia F, Dolezalova P, Biesen R, Shimizu M, Ullmann U, Mahmood A, Danquah A, Burillo E, Petrimpol M, Mallett S, Jamieson B, De Benedetti F. Emapalumab Treatment for Patients with Differing Presentations of Macrophage Activation Syndrome (MAS) Secondary to Still’s Disease: Results from a Pooled Analysis of Two Prospective Trials [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/emapalumab-treatment-for-patients-with-differing-presentations-of-macrophage-activation-syndrome-mas-secondary-to-stills-disease-results-from-a-pooled-analysis-of-two-prospective-trials/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/emapalumab-treatment-for-patients-with-differing-presentations-of-macrophage-activation-syndrome-mas-secondary-to-stills-disease-results-from-a-pooled-analysis-of-two-prospective-trials/
